The traditional Chinese medicine Qiliqiangxin in heart failure with reduced ejection fraction: a randomized, double-blind, placebo-controlled trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
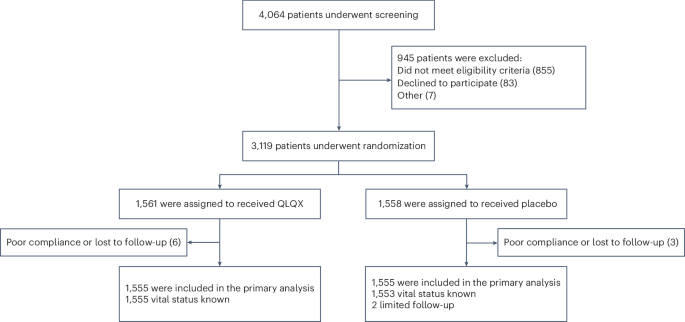
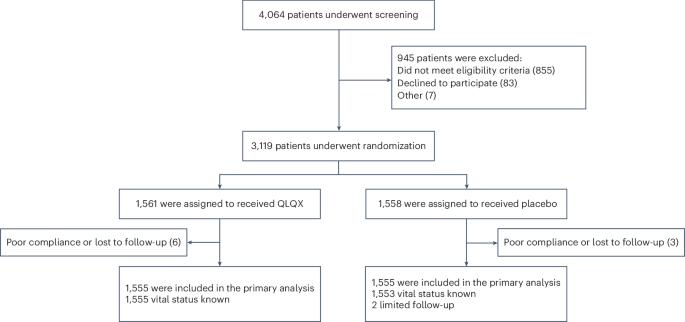
Previous findings have indicated the potential benefits of the Chinese traditional medicine Qiliqiangxin (QLQX) in heart failure. Here we performed a double-blind, randomized controlled trial to evaluate the efficacy and safety of QLQX in patients with heart failure and reduced ejection fraction (HFrEF). This multicenter trial, conducted in 133 hospitals in China, enrolled 3,110 patients with HFrEF with NT-proBNP levels of ≥450 pg ml−1 and left ventricular ejection fraction of ≤40%. Participants were randomized to receive either QLQX capsules or placebo (four capsules three times daily) alongside standard heart failure therapy. The trial met its primary outcome, which was a composite of hospitalization for heart failure and cardiovascular death: over a median follow-up of 18.3 months, the primary outcome occurred in 389 patients (25.02%) in the QLQX group and 467 patients (30.03%) in the placebo group (hazard ratio (HR), 0.78; 95% confidence interval (CI), 0.68−0.90; P < 0.001). In an analysis of secondary outcomes, the QLQX group showed reductions in both hospitalization for heart failure (15.63% versus 19.16%; HR, 0.76; 95% CI, 0.64−0.90; P = 0.002) and cardiovascular death (13.31% versus 15.95%; HR, 0.83; 95% CI, 0.68−0.996; P = 0.045) compared to the placebo group. All-cause mortality did not differ significantly between the two groups (HR, 0.84; 95% CI, 0.70−1.01; P = 0.058) and adverse events were also comparable between the groups. The results of this trial indicate that QLQX may improve clinical outcomes in patients with HFrEF when added to conventional therapy. ChiCTR registration: ChiCTR1900021929 . In a randomized controlled trial involving patients with heart failure with reduced ejection fraction, treatment with Qiliqiangxin, a traditional Chinese medicine derived from 11 types of plants, decreased the incidence of cardiac events, as compared to placebo.
中药芪蛭降糖丸治疗射血分数降低型心力衰竭:随机、双盲、安慰剂对照试验
以前的研究结果表明,中药七里香(QLQX)对心力衰竭有潜在疗效。在此,我们进行了一项双盲随机对照试验,以评估芪蛭降糖丸对射血分数降低型心力衰竭(HFrEF)患者的疗效和安全性。这项多中心试验在中国的 133 家医院进行,共招募了 3110 名 NT-proBNP 水平≥450 pg ml-1、左室射血分数≤40%的 HFrEF 患者。参与者被随机分配接受 QLQX 胶囊或安慰剂(每天三次,每次四粒),同时接受标准心衰治疗。试验达到了主要结果,即心衰住院和心血管死亡的综合结果:在18.3个月的中位随访期间,QLQX组有389名患者(25.02%)出现了主要结果,安慰剂组有467名患者(30.03%)出现了主要结果(危险比(HR),0.78;95%置信区间(CI),0.68-0.90;P< 0.001)。在次要结果分析中,与安慰剂组相比,QLQX 组的心衰住院率(15.63% 对 19.16%;HR,0.76;95% CI,0.64-0.90;P = 0.002)和心血管死亡率(13.31% 对 15.95%;HR,0.83;95% CI,0.68-0.996;P = 0.045)均有所下降。两组的全因死亡率没有明显差异(HR,0.84;95% CI,0.70-1.01;P = 0.058),两组的不良反应也相当。该试验结果表明,在常规治疗的基础上,QLQX可改善HFrEF患者的临床预后。ChiCTR注册:ChiCTR1900021929。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


