Development of Two Synthetic Routes to 4‐(N‐Boc‐N‐methylaminomethyl)benzaldehyde: A Versatile Starting Material in Pharmaceutical Synthesis
IF 2.8
4区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Two novel synthetic routes to 4‐(N‐Boc‐N‐methylaminomethyl)benzaldehyde, an important pharmaceutical starting material, were developed using terephthaloyl chloride and terephthalaldehyde. In the first approach, terephthaloyl chloride was converted to an ester amide. The treatment of the ester amide with LiAlH4 followed by protection of the resulting amine with tert‐butoxycarbonyl anhydride yielded the corresponding benzyl alcohol. The benzyl alcohol was oxidized to the aldehyde completing the first‐generation synthesis. The second approach utilized a one‐step protocol mono‐selective reductive amination of terephthalaldehyde with N‐Boc‐methylamine using chlorodimethylsilane. Both methods were scalable to 50 mmol and provided the desired aldehyde in a synthetically useful yield, demonstrating their practicality.
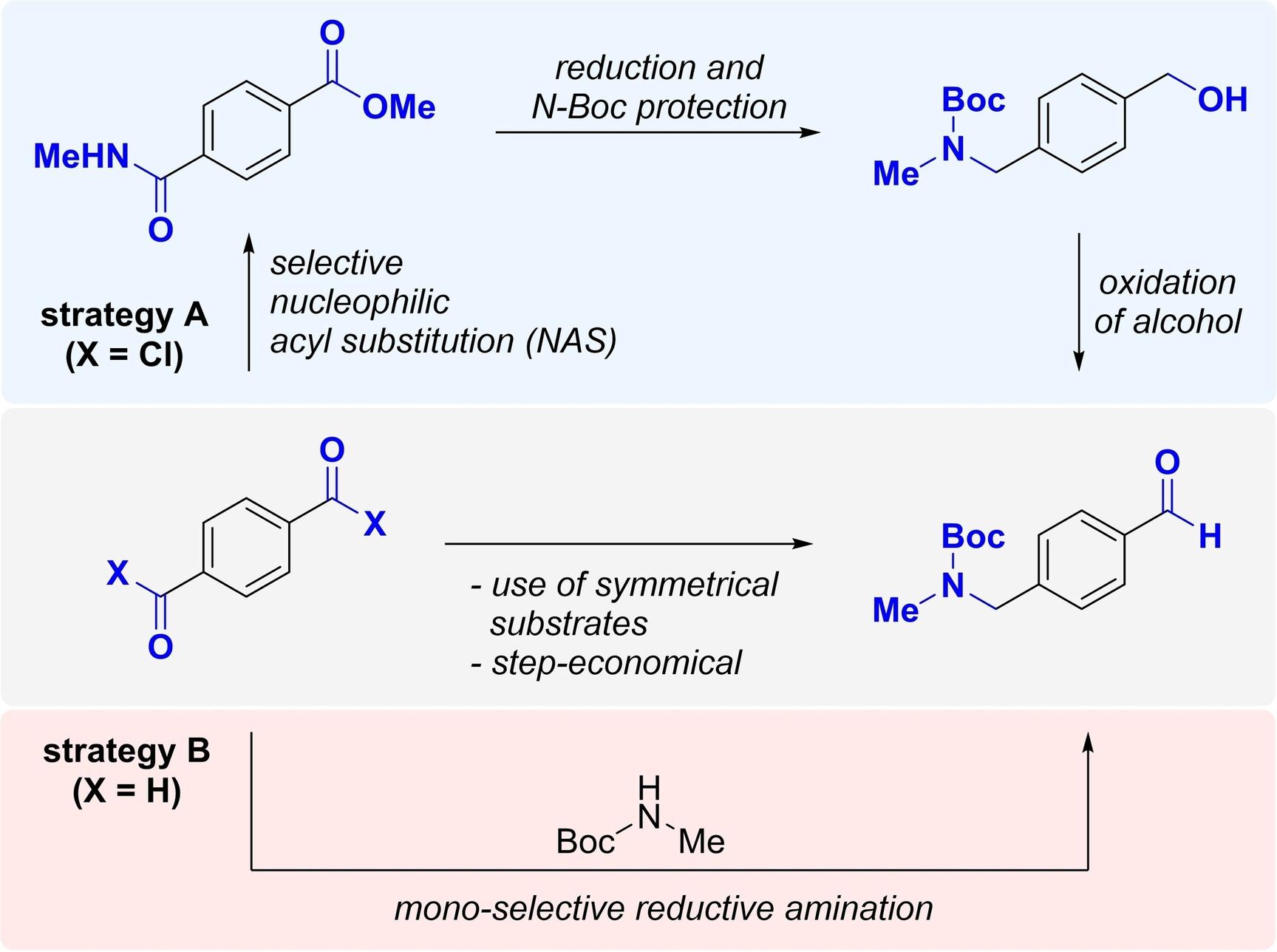
开发 4-(N-叔丁氧羰基-N-甲基氨基甲基)苯甲醛的两种合成路线:药物合成中的一种多功能起始原料
研究人员利用对苯二甲酰氯和对苯二甲醛,开发出了 4-(N-叔丁氧羰基-N-甲基氨基甲基)苯甲醛的两种新合成路线。在第一种方法中,对苯二甲酰氯被转化为酯酰胺。用 LiAlH4 处理酯酰胺,然后用叔丁氧羰基酸酐保护生成的胺,最后得到相应的苄醇。苄醇被氧化成醛,完成了第一代合成。第二种方法是利用氯二甲基硅烷一步法将对苯二甲醛与 N-叔丁氧羰基甲胺进行单选择性还原胺化。这两种方法都可以放大到 50 毫摩尔,并以合成有用的产率提供所需的醛,证明了它们的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Asian Journal of Organic Chemistry
CHEMISTRY, ORGANIC-
CiteScore
4.70
自引率
3.70%
发文量
372
期刊介绍:
Organic chemistry is the fundamental science that stands at the heart of chemistry, biology, and materials science. Research in these areas is vigorous and truly international, with three major regions making almost equal contributions: America, Europe and Asia. Asia now has its own top international organic chemistry journal—the Asian Journal of Organic Chemistry (AsianJOC)
The AsianJOC is designed to be a top-ranked international research journal and publishes primary research as well as critical secondary information from authors across the world. The journal covers organic chemistry in its entirety. Authors and readers come from academia, the chemical industry, and government laboratories.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


