LiOH-mediated crystallization regulating strategy enhancing electrochemical performance and structural stability of SiO anodes for lithium-ion batteries
Abstract
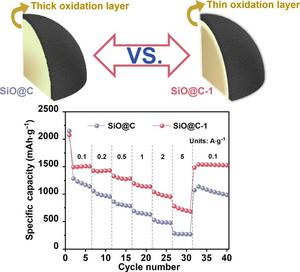
Silicon monoxide (SiO) is widely recognized as a promising anode material for next-generation lithium-ion batteries. Owing to its metastable amorphous structure, SiO exhibits a highly complex degree of crystallization at the microscopic level, which significantly influences its electrochemical behavior. As a consequence, accurately regulating the crystallization of SiO, and further establishing the relationship between crystallinity and electrochemical performance are very critical for SiO anodes. In this article, carbon-coated SiO materials with different crystallinity degrees were synthesized using lithium hydroxide monohydrate (LiOH·H2O) as a structural modifier to reveal this rule. Additionally, moderate amount of LiOH·H2O addition results in the forming of an oxygen-rich shell, which effectively inhibits the inward migration of oxygen atoms on the SiO surface and suppresses volume expansion. However, the crystallinity of SiO will gradually enhance and the crystalline phase appears with increasing the amount of LiOH·H2O, which will generate a deteriorative Li+ diffusion kinetic. After balancing the above two contradictions, a mass fraction of 1% LiOH·H2O for the additive yielded SiO@C-1, characterized by optimal crystallinity. SiO@C-1 demonstrates exceptional long-cycle stability with 74.8% capacity retention after 500 cycles at 1 A·g−1. Furthermore, it achieves a capacity retention of 52.2% even at a high density of 5 A·g−1. This study first reveals the relationship between SiO crystallinity and electrochemical performance, which efficiently guides the design of high-performance SiO anodes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


