Efficient hydrodeoxygenation of lignin-derived phenolic compounds under acid-free conditions over carbon-supported NiMo catalysts†
Abstract
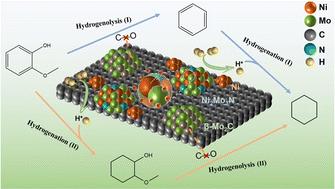
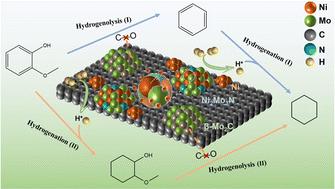
High-quality liquid biofuels can be produced from renewable lignin-derived phenolic compounds through an efficient hydrodeoxygenation (HDO) process in which the traditional catalysts usually include metal sites and acid sites that catalyze the hydrogenation and deoxygenation procedures respectively. This work presents a novel acid-free NixMoyN/C catalyst from the perspective of green chemistry providing a new pathway for HDO of lignin-derived phenolic compounds that involves hydrogenation deoxygenation and hydrogenolysis at the same time. A series of NixMoyN/C catalysts were prepared by varying the Ni : Mo molar ratio among which the Ni1Mo3N/C catalyst showed the best HDO performance. Guaiacol could be completely converted at 260 °C after 4 h with 95.8% cyclohexane selectivity. In addition a small amount of benzene could be obtained as a valuable fuel additive by-product by altering the conventional HDO reaction path. By shortening the reaction time benzene could be obtained as an intermediate product with a relative high selectivity. Based on the characterizations using XRD BET SEM TEM XPS H2-TPD and EPR, the results demonstrate that the multiple active components of the Ni1Mo3N/C catalyst allow it to efficiently catalyze the hydrogen activation and C–O bond cleavage even under acid-free conditions. The existence of the active phases of Ni Ni2Mo3N and β-Mo2C as well as the interaction between Ni and Mo metals together contributed toward efficient HDO performance. Not only for the various phenolic model compounds the feasibility of Ni1Mo3N/C catalysts for upgrading raw lignin oil was also demonstrated with the hydrocarbon content increasing from 5.7% to 88.4%. Notably arenes accounted for 18.2% of the hydrocarbon products which confirmed the occurrence of hydrogenolysis in the catalytic process. This work provides a novel route for the conversion of lignin-derived phenolic compounds to produce high-quality hydrocarbon liquid biofuels especially the direct production of arene components.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


