Unique expression and critical role of metallothionein 3 in the control of osteoclastogenesis and osteoporosis
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
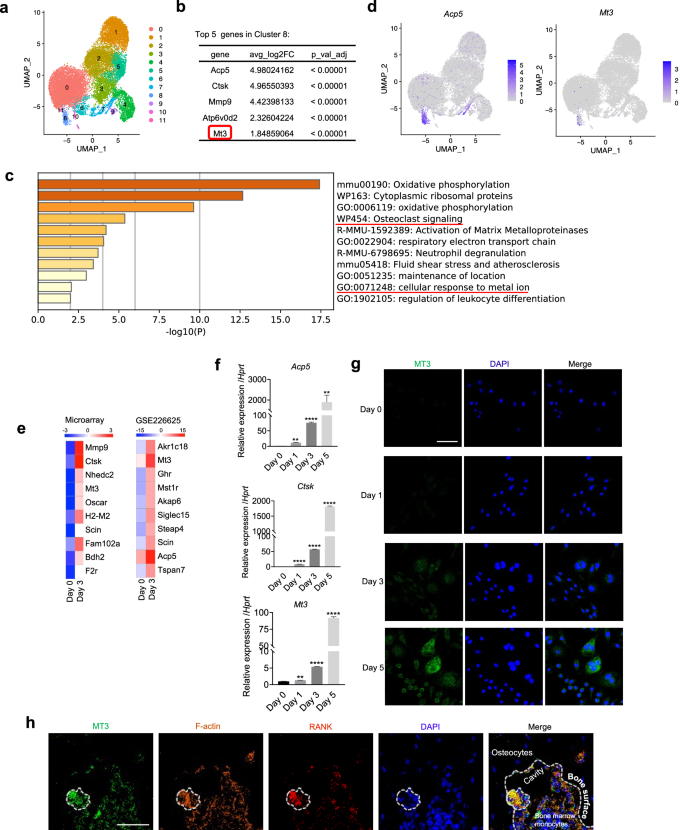
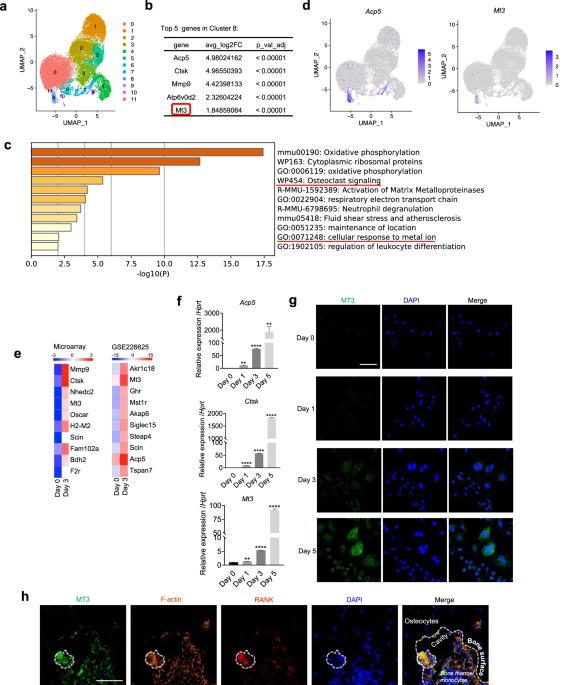
Bone homeostasis is maintained by an intricate balance between osteoclasts and osteoblasts, which becomes disturbed in osteoporosis. Metallothioneins (MTs) are major contributors in cellular zinc regulation. However, the role of MTs in bone cell regulation has remained unexplored. Single-cell RNA sequencing analysis discovered that, unlike the expression of other MT members, the expression of MT3 was unique to osteoclasts among various macrophage populations and was highly upregulated during osteoclast differentiation. This unique MT3 upregulation was validated experimentally and supported by ATAC sequencing data analyses. Downregulation of MT3 by gene knockdown or knockout resulted in excessive osteoclastogenesis and exacerbated bone loss in ovariectomy-induced osteoporosis. Transcriptome sequencing of MT3 knockdown osteoclasts and gene set enrichment analysis indicated that the oxidative stress and redox pathways were enriched, which was verified by MT3-dependent regulation of reactive oxygen species (ROS). In addition, MT3 deficiency increased the transcriptional activity of SP1 in a manner dependent on intracellular zinc levels. This MT3-zinc-SP1 axis was crucial for the control of osteoclasts, as zinc chelation and SP1 knockdown abrogated the promotion of SP1 activity and osteoclastogenesis by MT3 deletion. Moreover, SP1 bound to the NFATc1 promoter, and overexpression of an inactive SP1 mutant negated the effects of MT3 deletion on NFATc1 and osteoclastogenesis. In conclusion, MT3 plays a pivotal role in controlling osteoclastogenesis and bone metabolism via dual axes involving ROS and SP1. The present study demonstrated that MT3 elevation is a potential therapeutic strategy for osteolytic bone disorders, and it established for the first time that MT3 is a crucial bone mass regulator. Bone diseases such as osteoporosis often result from imbalances in bone remodeling, a process involving bone breakdown by cells called osteoclasts and formation by cells called osteoblasts. This study examines the role of Metallothionein 3, a protein that binds to zinc, in osteoclasts. Using a mix of single-cell RNA sequencing database and knockout mouse models, the study investigates how MT3 affects osteoclast development and activity. The researchers used various methods, including gene knockdown and overexpression techniques, to alter MT3 levels in cells and observed the effects on osteoclast formation and bone breakdown. The results indicate that MT3 inhibits osteoclast development and decreases bone loss, suggesting its potential as a treatment target for bone diseases. The study concludes that MT3 plays a crucial role in bone remodeling by controlling osteoclast activity. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
金属硫蛋白 3 在控制破骨细胞生成和骨质疏松症中的独特表达和关键作用。
骨平衡由破骨细胞和成骨细胞之间错综复杂的平衡来维持,而骨质疏松症会破坏这种平衡。金属硫蛋白(MTs)是细胞锌调节的主要成分。然而,MTs 在骨细胞调控中的作用仍未得到探索。单细胞 RNA 测序分析发现,与其他 MT 成员的表达不同,在各种巨噬细胞群中,破骨细胞独有 MT3 的表达,并且在破骨细胞分化过程中高度上调。这种独特的 MT3 上调得到了实验验证和 ATAC 测序数据分析的支持。在卵巢切除术诱导的骨质疏松症中,通过基因敲除或基因敲除下调 MT3 会导致过度的破骨细胞生成并加剧骨质流失。MT3基因敲除破骨细胞的转录组测序和基因组富集分析表明,氧化应激和氧化还原通路被富集,这一点通过MT3对活性氧(ROS)的依赖性调控得到了验证。此外,MT3 的缺乏增加了 SP1 的转录活性,其方式依赖于细胞内的锌水平。MT3-锌-SP1轴对破骨细胞的控制至关重要,因为锌螯合和SP1敲除会减弱MT3缺失对SP1活性和破骨细胞生成的促进作用。此外,SP1与NFATc1启动子结合,过表达无活性的SP1突变体可抵消MT3缺失对NFATc1和破骨细胞生成的影响。总之,MT3通过涉及ROS和SP1的双轴在控制破骨细胞生成和骨代谢中起着关键作用。本研究表明,MT3的升高是溶骨性骨病的一种潜在治疗策略,并首次证实了MT3是一种关键的骨量调节因子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


