Low-level brain somatic mutations in exonic regions are collectively implicated in autism with germline mutations in autism risk genes
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
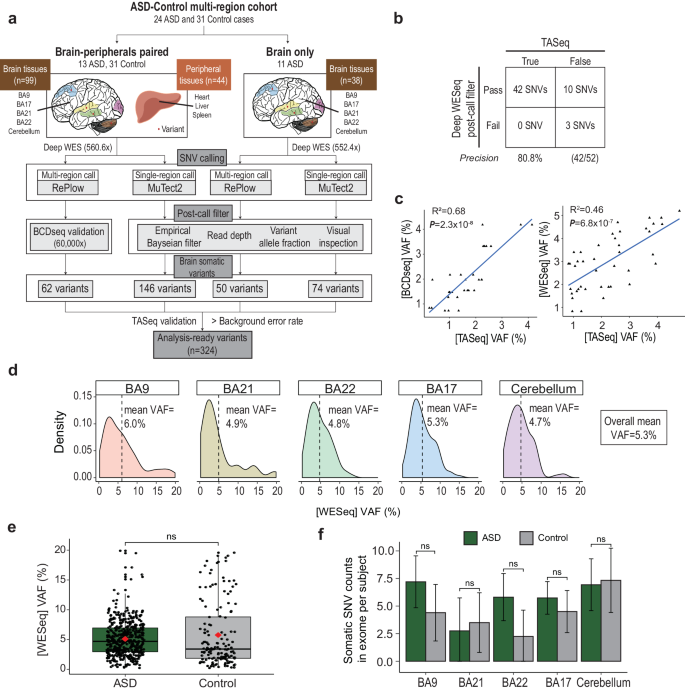
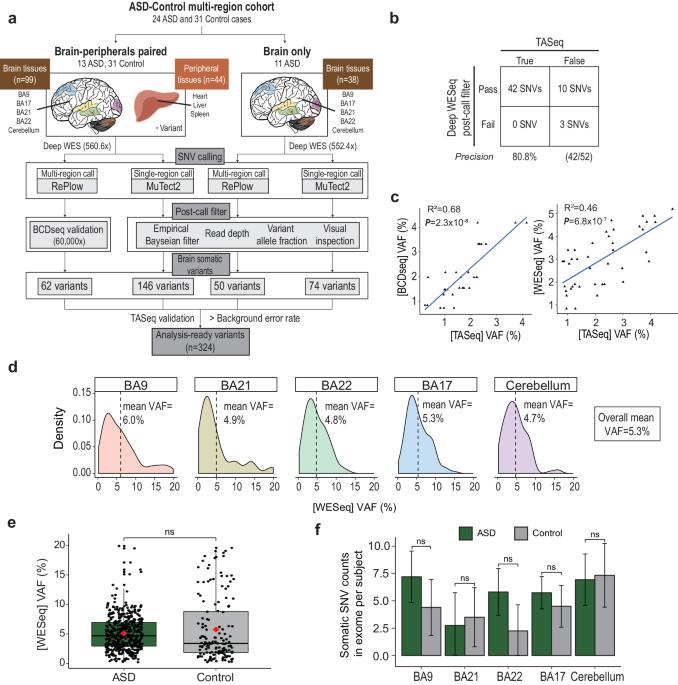
Low-level somatic mutations in the human brain are implicated in various neurological disorders. The contribution of low-level brain somatic mutations to autism spectrum disorder (ASD), however, remains poorly understood. Here, we performed high-depth exome sequencing with an average read depth of 559.3x in 181 cortical, cerebellar, and peripheral tissue samples to identify brain somatic single nucleotide variants (SNVs) in 24 ASD subjects and 31 controls. We detected ~2.4 brain somatic SNVs per exome per single brain region, with a variant allele frequency (VAF) as low as 0.3%. The mutational profiles, including the number, signature, and type, were not significantly different between the ASD patients and controls. Intriguingly, when considering genes with low-level brain somatic SNVs and ASD risk genes with damaging germline SNVs together, the merged set of genes carrying either somatic or germline SNVs in ASD patients was significantly involved in ASD-associated pathophysiology, including dendrite spine morphogenesis (p = 0.025), mental retardation (p = 0.012), and intrauterine growth retardation (p = 0.012). Additionally, the merged gene set showed ASD-associated spatiotemporal expression in the early and mid-fetal cortex, striatum, and thalamus (all p < 0.05). Patients with damaging mutations in the merged gene set had a greater ASD risk than did controls (odds ratio = 3.92, p = 0.025, 95% confidence interval = 1.12–14.79). The findings of this study suggest that brain somatic SNVs and germline SNVs may collectively contribute to ASD-associated pathophysiology. Autism Spectrum Disorder is a complex condition influenced by various genetic factors, including inherited traits and new changes in genes. This study investigates the role of low-level brain somatic mutations in ASD. The researchers analyzed brain tissues from deceased individuals, both with and without ASD, using high-depth whole-exome sequencing. The results showed that low-level brain somatic mutations, along with inherited genetic variations, contribute to ASD’s genetic makeup. These mutations were found in genes linked to brain development and function. The study emphasizes the need to consider both inherited and somatic mutations to understand ASD’s genetic complexity. Researchers conclude that the interaction between somatic and inherited mutations is crucial in ASD, providing new insights into its genetic basis. This study enhances our understanding of ASD’s genetic diversity and suggests a multifaceted genetic contribution to the disorder. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
外显子区域的低水平脑体细胞突变与自闭症风险基因的种系突变共同牵涉到自闭症。
人脑中的低水平体细胞突变与各种神经系统疾病有关。然而,人们对低水平大脑体细胞突变对自闭症谱系障碍(ASD)的影响仍然知之甚少。在这里,我们对 181 份皮层、小脑和外周组织样本进行了平均读取深度为 559.3 倍的高深度外显子测序,以鉴定 24 名 ASD 受试者和 31 名对照者的脑体细胞单核苷酸变异(SNVs)。我们在每个脑区的每个外显子组检测到约 2.4 个脑部体细胞 SNV,变异等位基因频率 (VAF) 低至 0.3%。ASD患者和对照组的突变特征,包括数量、特征和类型,均无显著差异。耐人寻味的是,当把低水平脑部体细胞SNV基因和具有破坏性种系SNV基因的ASD风险基因放在一起考虑时,ASD患者中携带体细胞或种系SNV基因的合并基因集明显参与了ASD相关的病理生理学,包括树突棘形态发生(p = 0.025)、智力迟钝(p = 0.012)和宫内生长迟缓(p = 0.012)。此外,合并后的基因组在胎儿早期和中期皮层、纹状体和丘脑中显示出与 ASD 相关的时空表达(均 p = 0.025)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


