Structural switch in acetylcholine receptors in developing muscle
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
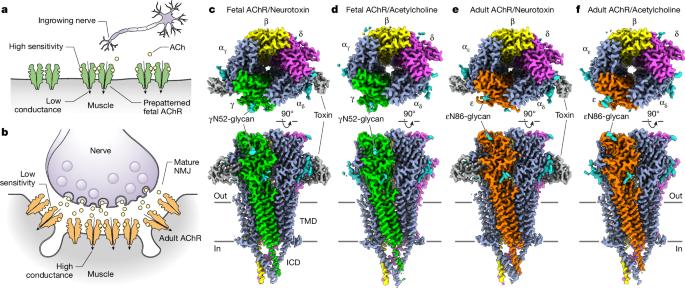
During development, motor neurons originating in the brainstem and spinal cord form elaborate synapses with skeletal muscle fibres1. These neurons release acetylcholine (ACh), which binds to nicotinic ACh receptors (AChRs) on the muscle, initiating contraction. Two types of AChR are present in developing muscle cells, and their differential expression serves as a hallmark of neuromuscular synapse maturation2–4. The structural principles underlying the switch from fetal to adult muscle receptors are unknown. Here, we present high-resolution structures of both fetal and adult muscle nicotinic AChRs, isolated from bovine skeletal muscle in developmental transition. These structures, obtained in the absence and presence of ACh, provide a structural context for understanding how fetal versus adult receptor isoforms are tuned for synapse development versus the all-or-none signalling required for high-fidelity skeletal muscle contraction. We find that ACh affinity differences are driven by binding site access, channel conductance is tuned by widespread surface electrostatics and open duration changes result from intrasubunit interactions and structural flexibility. The structures further reveal pathogenic mechanisms underlying congenital myasthenic syndromes. Structures of fetal and adult muscle acetylcholine receptors reveal a developmental switch that alters channel biophysics and pharmacology to enable neuromuscular junction maturation, uncovering pathogenic mechanisms underlying congenital myasthenic syndromes.
发育中肌肉中乙酰胆碱受体的结构变化
在发育过程中,起源于脑干和脊髓的运动神经元与骨骼肌纤维形成复杂的突触1。这些神经元释放乙酰胆碱(ACh),乙酰胆碱与肌肉上的烟碱型 ACh 受体(AChRs)结合,引发肌肉收缩。发育中的肌肉细胞中存在两种类型的 AChR,它们的不同表达是神经肌肉突触成熟的标志2-4。从胎儿到成年肌肉受体转换的结构原理尚不清楚。在这里,我们展示了从处于发育过渡期的牛骨骼肌中分离出的胎儿和成肌烟碱 AChR 的高分辨率结构。这些结构是在无 ACh 和有 ACh 的情况下获得的,为了解胎儿和成年肌肉受体异构体如何针对突触发育和高保真骨骼肌收缩所需的全或无信号进行调整提供了结构背景。我们发现,乙酰胆碱亲和力的差异是由结合位点访问驱动的,通道电导是由广泛的表面静电调整的,开放持续时间的变化是由亚基内相互作用和结构灵活性导致的。这些结构进一步揭示了先天性肌无力综合征的致病机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


