Histone serotonylation regulates ependymoma tumorigenesis
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Bidirectional communication between tumours and neurons has emerged as a key facet of the tumour microenvironment that drives malignancy1,2. Another hallmark feature of cancer is epigenomic dysregulation, in which alterations in gene expression influence cell states and interactions with the tumour microenvironment3. Ependymoma (EPN) is a paediatric brain tumour that relies on epigenomic remodelling to engender malignancy4,5; however, how these epigenetic mechanisms intersect with extrinsic neuronal signalling during EPN tumour progression is unknown. Here we show that the activity of serotonergic neurons regulates EPN tumorigenesis, and that serotonin itself also serves as an activating modification on histones. We found that inhibiting histone serotonylation blocks EPN tumorigenesis and regulates the expression of a core set of developmental transcription factors. High-throughput, in vivo screening of these transcription factors revealed that ETV5 promotes EPN tumorigenesis and functions by enhancing repressive chromatin states. Neuropeptide Y (NPY) is one of the genes repressed by ETV5, and its overexpression suppresses EPN tumour progression and tumour-associated network hyperactivity through synaptic remodelling. Collectively, this study identifies histone serotonylation as a key driver of EPN tumorigenesis, and also reveals how neuronal signalling, neuro-epigenomics and developmental programs are intertwined to drive malignancy in brain cancer. Serotonin has a role in ependymoma tumorigenesis through modifying histones and thereby regulating key transcription factors and activating specific oncogenic transcriptional networks in brain cells.
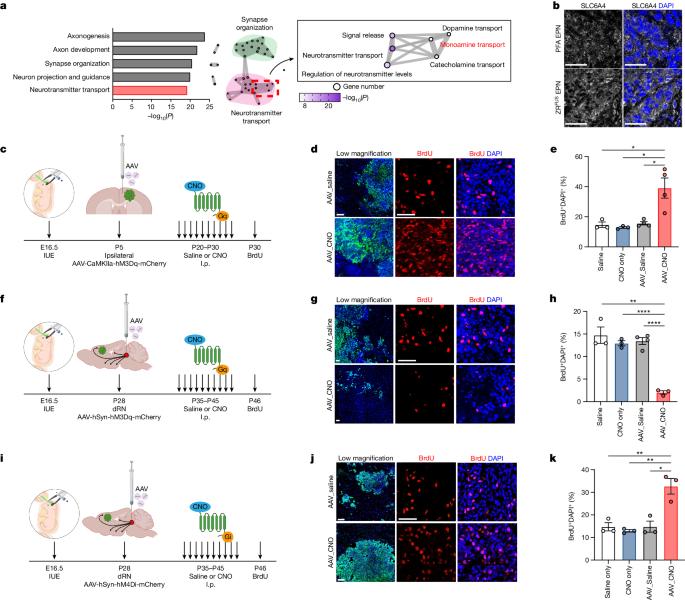
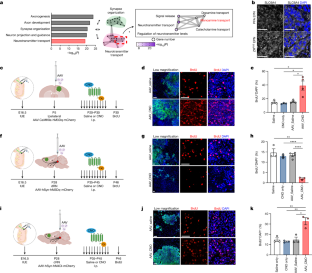
组蛋白血清素化调控附肢瘤的肿瘤发生。
肿瘤与神经元之间的双向交流已成为肿瘤微环境的一个关键方面,它是恶性肿瘤的驱动因素1,2。癌症的另一个标志性特征是表观基因组失调,其中基因表达的改变会影响细胞状态以及与肿瘤微环境的相互作用3。脑上皮瘤(EPN)是一种依靠表观基因组重塑产生恶性肿瘤的儿科脑肿瘤4,5;然而,这些表观遗传学机制如何在 EPN 肿瘤进展过程中与外在神经元信号相互交织尚不清楚。在这里,我们发现血清素能神经元的活动调控 EPN 肿瘤的发生,而血清素本身也是组蛋白的激活修饰。我们发现,抑制组蛋白血清素化能阻止 EPN 肿瘤发生,并调节一组核心发育转录因子的表达。对这些转录因子的高通量体内筛选发现,ETV5能促进EPN肿瘤发生,并通过增强抑制性染色质状态发挥作用。神经肽Y(NPY)是被ETV5抑制的基因之一,它的过表达通过突触重塑抑制了EPN肿瘤的进展和肿瘤相关网络的过度活跃。总之,这项研究发现组蛋白5-羟色胺化是EPN肿瘤发生的关键驱动因素,同时也揭示了神经元信号、神经表观基因组学和发育程序是如何交织在一起驱动脑癌恶变的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


