Peptide array screening with anti-GLP-1 monoclonal antibody: Discovery of cysteine-containing DPP-IV inhibitory peptides
Abstract
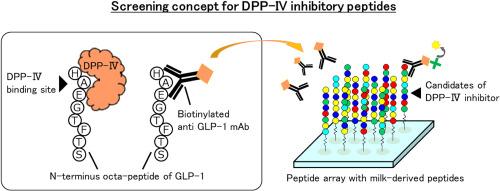
Inhibition of dipeptidyl peptidase IV (DPP-IV) is an effective pharmacotherapy for the management of type 2 diabetes. Recent findings have suggested that various dietary proteins can serve as precursors to peptides that inhibit DPP-IV. Although several DPP-IV inhibitory peptides derived from food materials have been reported, more effective inhibitory peptides remain to be discovered. This study aimed to identify potent DPP-IV inhibitory peptides that earlier approaches had overlooked by employing a screening method that combined peptide arrays and neutralizing antibodies. Octa-peptides covering the complete amino acid sequences of four casein proteins and two whey proteins were synthesized on arrays via a solid-phase method. These peptides were then reacted with a monoclonal antibody specifically engineered to recognize glucagon-like peptide 1 (GLP-1), a substrate of DPP-IV. The variable region of the anti-GLP-1 monoclonal antibody is utilized to mimic the substrate-binding region of DPP-IV, enabling the antibody to bind to peptides that interact with DPP-IV. Based on this feature, 26 peptides were selected as DPP-IV inhibitory peptide candidates, 11 of which showed strong DPP-IV inhibitory activity. Five of these peptides consistently contained cysteines positioned two to four residues from the N-terminus. Treatment with disulfide formation decreased the DPP-IV inhibitory activity of these cysteine-containing peptides, while the inhibitory activity of α-lactalbumin hydrolysates increased with reducing treatment. These results revealed that the thiol group is important for DPP-IV inhibitory activity. This study provides a useful screen for DPP-IV inhibitory peptides and indicates the importance of reductive cysteine residues within DPP-IV inhibitory peptides.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


