Overcoming Barriers to Remission in Severe Eosinophilic Asthma: Two-Year Real-World Data With Benralizumab
Abstract
Background
Benralizumab has been reported to lead to clinical remission of severe eosinophilic asthma (SEA) at 1 year in some patients. However, whether this is maintained over a longer term remains unclear. Additionally, the impact of pulmonary and extrapulmonary comorbidities on the ability to meet remission is poorly understood.
Methods
Clinical outcomes including remission of SEA with benralizumab at 1 and 2 years were assessed retrospectively in a real-world UK multi-centre severe asthma cohort. The presence of clinically relevant pulmonary and extrapulmonary comorbidities associated with respiratory symptoms was recorded. Analyses to identify factors associated with the ability to meet remission were performed.
Results
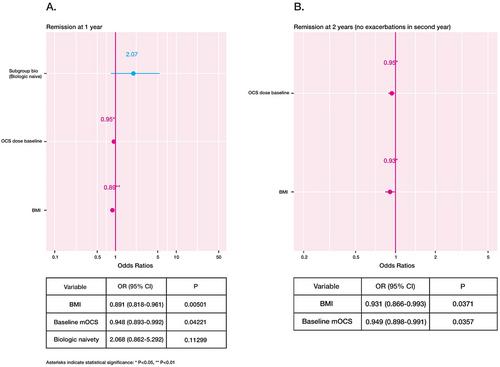
In total, 276 patients with SEA treated with benralizumab including 113 patients who had switched from a previous biologic to benralizumab were included. Overall, clinical remission was met in 17% (n = 31/186) and 32% (n = 43/133) of patients at 1 and 2 years, respectively. This increased to 28% at 1 year and 49% at 2 years once patients with pulmonary and/or extrapulmonary comorbidities were excluded. Body mass index (BMI) and maintenance OCS (mOCS) use demonstrated a negative association with clinical remission at 1 (BMI: OR: 0.89, 95% CI: 0.82–0.96, p < 0.01; mOCS: OR: 0.94, 95% CI: 0.89–0.99, p < 0.05) and 2 years (BMI: OR: 0.93, 95% CI: 0.87–0.99, p < 0.05; mOCS: OR: 0.95, 95% CI: 0.89–0.99, p < 0.05).
Conclusions
In this long-term, real-world study, patients with SEA demonstrated the ability to meet and sustain clinical remission when treated with benralizumab. The presence of comorbidities including obesity, which are known to be independently associated with respiratory symptoms, reduced the likelihood of meeting clinical remission.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


