Quinone-Initiated Photocatalytic Enantioselective Giese Radical Addition with Ethers, Thioethers, Amines, and Alkanes
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Photocatalytic enantioselective Giese radical addition with inert C(sp3)–H bonds represents a highly efficient and economically favorable approach to synthesizing diverse value-added chiral molecules from abundant feedstock. Herein, we disclose a quinone-initiated photocatalytic asymmetric Giese radical addition of α-substituted acrylamides with inert C(sp3)–H bonds by applying simple quinones as HAT photocatalysts in combination with a chiral N,N′-dioxide/praseodymium(III) catalyst. A wide array of ethers, thioethers, selenide, amines, and alkanes can smoothly transform into the corresponding chiral α-aryl amide derivatives with satisfactory enantioselectivities (68 examples, up to 95% ee) under mild conditions. Based on spectroscopy studies and control experiments, a quinone-initiated HAT catalytic cycle was proposed, and DFT calculations revealed that the interaction between quinone and chiral Lewis acid was essential for enantio-induction in the asymmetric back hydrogen atom transfer process.
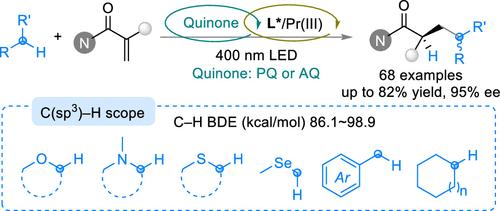
醌引发的光催化对映体选择性吉斯辐射与醚、硫醚、胺和烷的加成反应
用惰性 C(sp3)-H 键进行光催化对映体选择性 Giese 自由基加成是利用丰富的原料合成多种高附加值手性分子的一种高效且经济的方法。在此,我们揭示了一种由醌引发的α-取代的丙烯酰胺与惰性 C(sp3)-H 键的不对称 Giese 自由基加成的光催化方法,其方法是将简单的醌作为 HAT 光催化剂与手性 N,N′-二氧化物/镨(III)催化剂结合使用。在温和的条件下,各种醚、硫醚、硒化物、胺和烷烃都能顺利转化为相应的手性 α-芳基酰胺衍生物,并具有令人满意的对映选择性(68 个实例,高达 95% ee)。基于光谱研究和对照实验,提出了一种由醌引发的 HAT 催化循环,DFT 计算表明,在不对称氢原子反向转移过程中,醌与手性路易斯酸之间的相互作用对对映体诱导至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


