Synthesis of Lenacapavir Sodium: Active Pharmaceutical Ingredient Process Development and Scale-up
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Lenacapavir sodium (GS-6207-02) is a first-in-class HIV capsid inhibitor. Process development of the four-step final assembly of lenacapavir sodium from four synthetic intermediates is described here. A bis-bromopyridine core is sequentially subjected to an alkynylation, an amide coupling with a chiral pyrazole carboxylic acid, and a Suzuki cross-coupling with an indazole boronic ester. The final step is a telescoped bis-methanesulfonylation and hydrolysis to yield the API. This report highlights experimental work on the final assembly sequence to establish robust processing conditions, minimize process mass intensity, control impurity formation, understand impurity purge, and enable large-scale manufacturing of lenacapavir sodium.
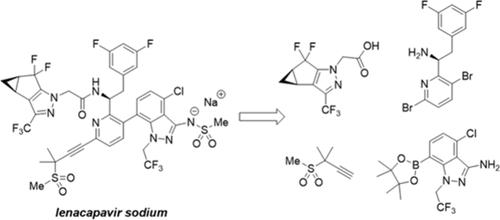
来那卡巴韦钠的合成:活性药物成分工艺开发与放大
来那卡韦钠(GS-6207-02)是第一类HIV囊膜抑制剂。本文介绍了来那卡韦钠由四种合成中间体经四步最终组装的工艺开发过程。双溴吡啶核心依次经过炔化、与手性吡唑羧酸的酰胺偶联以及与吲唑硼酸酯的铃木交叉偶联。最后一步是伸缩双甲磺酰化和水解,生成原料药。本报告重点介绍了最终装配序列的实验工作,以建立稳健的加工条件,最大限度地降低加工质量强度,控制杂质形成,了解杂质净化,并实现来那卡韦钠的大规模生产。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


