Nickel-Catalyzed Reductive Decarboxylative Coupling of Diacyl Peroxides with Aryl/Vinyl Halides
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
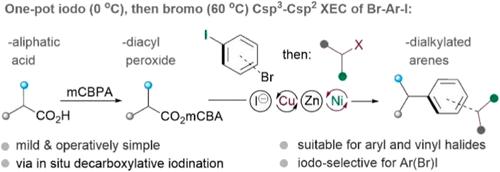
In this work, we utilized alkyl diacyl peroxides as the decarboxylative substrates, which are derived from the condensation of readily available alkyl acids with mCPBA, to couple with a variety of aryl and vinyl halides under a Ni-catalyzed cross-electrophile coupling platform, effectively affording C(sp3)–C(sp2) bonds. The method stresses the high compatibility of alkyl diacyl peroxides as strong oxidants with the Ni/Zn-mediated reductive conditions. With assistance of LiI and catalytic CuI, the kinetic labile alkyl diacyl peroxide underwent facile in situ Barton-decarboxylative iodination to afford alkyl iodide as the actual coupling partners. The present method showcases a broad substrate scope for both alkyl acids and aryl/vinyl electrophiles. It offers the advantage of iodo-selectivity for the alkylation of aryl iodides, even those bearing highly competing bromine groups, including the challenging vicinal bromoiodobenzene. Consequently, it enables the development of a one-pot selective dialkylation process.
镍催化的二酰过氧化物与芳基/乙烯基卤化物的还原脱羧偶联反应
在这项工作中,我们利用烷基二酰过氧化物作为脱羧底物,在镍催化的交叉亲电偶联平台下与多种芳基和乙烯基卤化物偶联,有效地生成了 C(sp3)-C(sp2)键。该方法强调了作为强氧化剂的烷基二酰过氧化物与镍/锌介导的还原条件的高度兼容性。在 LiI 和催化 CuI 的帮助下,动力学上易变的烷基二酰过氧化物进行了简便的原位巴顿-脱羧碘化反应,生成了烷基碘化物作为实际的偶联剂。本方法展示了烷基酸和芳基/乙烯基亲电体的广泛底物范围。它在芳基碘化物的烷基化方面具有碘选择性优势,即使是那些带有高度竞争性溴基团的芳基碘化物,包括具有挑战性的邻位溴碘苯。因此,它能够开发出一种单锅选择性二烷基化工艺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


