Distinct Intercalation and Conduction Behaviors within an Isostructural Series Ba5R2Al2SnO13
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The series Ba5R2Al2SnO13 (R = In, Y, Er, Ho, Tb) has been synthesized and structurally characterized by X-ray and neutron powder diffraction. All members have oxygen-deficient 10-layer hexagonal (10H) perovskite-type structures at high temperature and gain mass on cooling equivalent to ∼0.5 oxygen atoms per formula unit, observed by both thermogravimetric analysis and the occupancy of a vacant site in the oxygen substructure refined against neutron powder diffraction data. The origin of this mass gain varies with R: for R = In, Y, Er, and Ho, it is due to water uptake via a hydroxylation mechanism to form Ba5R2Al2SnO13.xH2O (x ≤ 0.5), with OH– ions occupying the vacant site and the other proton forming a second OH– in the oxygen substructure; while for R = Tb, it due to the oxidation of Tb3+ to Tb4+, with O2– ions occupying the vacant site. These chemico-structural differences are consistent with the measured conductivity behavior of the samples, whereby Ba5Er2Al2SnO13 is a proton conductor in air at moderate temperatures (∼10–4 S cm–1 at 500 °C) while Ba5Tb2Al2SnO13 is a mixed oxide ionic and electronic conductor. These differences were further confirmed by X-ray absorption spectroscopy and corroborated by quasielastic neutron scattering.
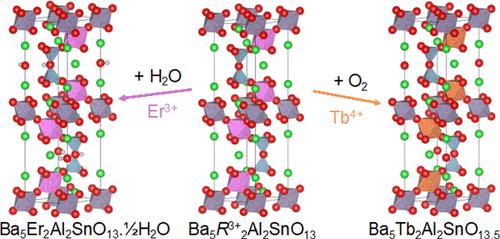
等结构系列 Ba5R2Al2SnO13 中不同的互钙化和传导行为
我们合成了一系列 Ba5R2Al2SnO13(R = In、Y、Er、Ho、Tb),并通过 X 射线和中子粉末衍射对其进行了结构表征。所有成员在高温下都具有缺氧的 10 层六边形(10H)包晶型结构,并且在冷却过程中质量增加,相当于每个公式单位增加了 ∼0.5 个氧原子,这是通过热重分析和根据中子粉末衍射数据改进的氧亚结构中一个空位的占据情况观察到的。质量增加的原因随 R 的不同而变化:对于 R = In、Y、Er 和 Ho,是由于通过羟基化机制吸水形成 Ba5R2Al2SnO13.xH2O (x ≤ 0.5),OH- 离子占据空位,另一个质子在氧亚结构中形成第二个 OH-;而对于 R = Tb,是由于 Tb3+ 氧化成 Tb4+,O2- 离子占据空位。这些化学结构上的差异与所测量到的样品导电行为一致,即 Ba5Er2Al2SnO13 在中等温度下是空气中的质子导体(500 °C 时为 ∼10-4 S cm-1),而 Ba5Tb2Al2SnO13 则是离子和电子混合氧化物导体。X 射线吸收光谱进一步证实了这些差异,准弹性中子散射也证实了这一点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


