Enhancing Water Tolerance and N2 Selectivity in NH3–SCR Catalysts by Protecting Mn Oxide Nanoparticles in a Silicalite-1 Layer
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Mn-based catalysts are promising candidates for eliminating harmful nitrogen oxides (NOx) via selective catalytic reduction with ammonia (NH3–SCR) due to their inherent strong redox abilities. However, poor water tolerance and low N2 selectivity are still the main limitations for practical applications. Herein, we succeeded in preparing an active catalyst for NH3–SCR with improved water tolerance and N2 selectivity based on protecting MnOx with a secondary growth of a hydrophobic silicalite-1. This protection suppressed catalyst deactivation by water adsorption. Interestingly, impregnating MnOx on MesoTS-1 followed by silicalite-1 protection allowed for a higher dispersion of MnOx species, thus increasing the concentration of acid sites. Consequently, the level of N2O formation is decreased. These improvements resulted in a broader operating temperature of NOx conversion and a modification of the NH3–SCR mechanism. Diffuse reflectance infrared Fourier transform spectroscopy analysis revealed that unprotected Mn/MesoTS-1 mainly followed the Eley–Rideal mechanism, while Mn/MesoTS-1@S1 followed both Langmuir–Hinshelwood and Eley–Rideal mechanisms.
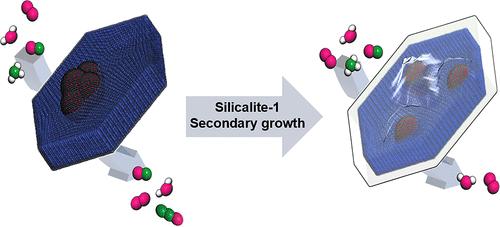
通过在硅胶-1 层中保护氧化锰纳米颗粒提高 NH3-SCR 催化剂的耐水性和 N2 选择性
锰基催化剂因其固有的强氧化还原能力,有望通过氨的选择性催化还原(NH3-SCR)消除有害的氮氧化物(NOx)。然而,耐水性差和 N2 选择性低仍然是实际应用的主要限制因素。在此,我们利用疏水性硅铝酸盐-1 的二次生长保护氧化锰,成功制备出一种具有更好耐水性和 N2 选择性的 NH3-SCR 活性催化剂。这种保护可抑制催化剂因吸附水而失活。有趣的是,将氧化锰浸渍在 MesoTS-1 上,然后用硅铝酸盐-1 进行保护,可以提高氧化锰的分散性,从而增加酸性位点的浓度。因此,N2O 的形成水平降低了。这些改进提高了氮氧化物转化的工作温度,并改变了 NH3-SCR 机制。漫反射红外傅立叶变换光谱分析显示,未受保护的 Mn/MesoTS-1 主要遵循 Eley-Rideal 机制,而 Mn/MesoTS-1@S1 则同时遵循 Langmuir-Hinshelwood 和 Eley-Rideal 机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


