Prevention and treatment of peri-implant fibrosis by functionally inhibiting skeletal cells expressing the leptin receptor
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
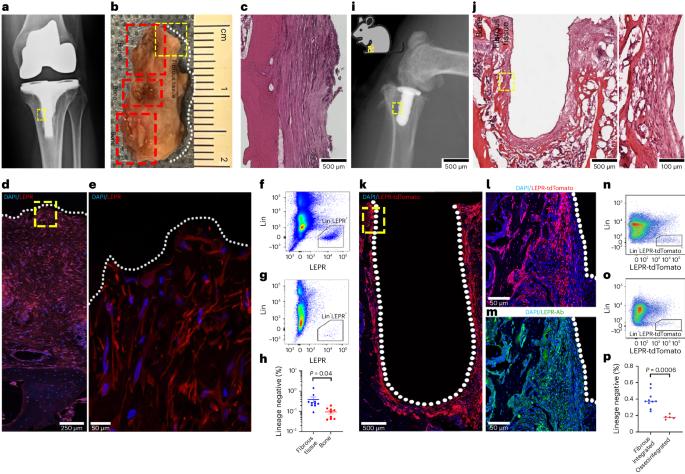
The cellular and molecular mediators of peri-implant fibrosis—a most common reason for implant failure and for surgical revision after the replacement of a prosthetic joint—remain unclear. Here we show that peri-implant fibrotic tissue in mice and humans is largely composed of a specific population of skeletal cells expressing the leptin receptor (LEPR) and that these cells are necessary and sufficient to generate and maintain peri-implant fibrotic tissue. In a mouse model of tibial implantation and osseointegration that mimics partial knee arthroplasty, genetic ablation of LEPR+ cells prevented peri-implant fibrosis and the implantation of LEPR+ cells from peri-implant fibrotic tissue was sufficient to induce fibrosis in secondary hosts. Conditional deletion of the adhesion G-protein-coupled receptor F5 (ADGRF5) in LEPR+ cells attenuated peri-implant fibrosis while augmenting peri-implant bone formation, and ADGRF5 inhibition by the intra-articular or systemic administration of neutralizing anti-ADGRF5 in the mice prevented and reversed peri-implant fibrosis. Pharmaceutical agents that inhibit the ADGRF5 pathway in LEPR+ cells may be used to prevent and treat peri-implant fibrosis. The generation and maintenance of peri-implant fibrotic tissue requires skeletal cells expressing the leptin receptor and can be prevented and reversed via the functional inhibition of these cells.
通过对表达瘦素受体的骨骼细胞进行功能抑制,预防和治疗种植体周围纤维化
种植体周围纤维化是导致种植失败和假体关节置换后手术翻修的最常见原因,但其细胞和分子介导因素仍不清楚。在这里,我们发现小鼠和人类的假体周围纤维化组织主要由表达瘦素受体(LEPR)的特定骨骼细胞群组成,这些细胞是产生和维持假体周围纤维化组织的必要和充分条件。在模拟部分膝关节置换术的胫骨植入和骨结合小鼠模型中,LEPR+细胞的基因消减可防止植入物周围纤维化,而植入物周围纤维化组织中的LEPR+细胞足以诱导继发宿主纤维化。在LEPR+细胞中条件性缺失粘附G蛋白偶联受体F5(ADGRF5)可减轻种植体周围纤维化,同时增强种植体周围骨形成,通过在小鼠关节内或全身给予中和性抗ADGRF5抑制ADGRF5可预防和逆转种植体周围纤维化。抑制 LEPR+ 细胞中 ADGRF5 通路的药物可用于预防和治疗种植体周围纤维化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


