High-resolution yeast actin structures indicate the molecular mechanism of actin filament stiffening by cations
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
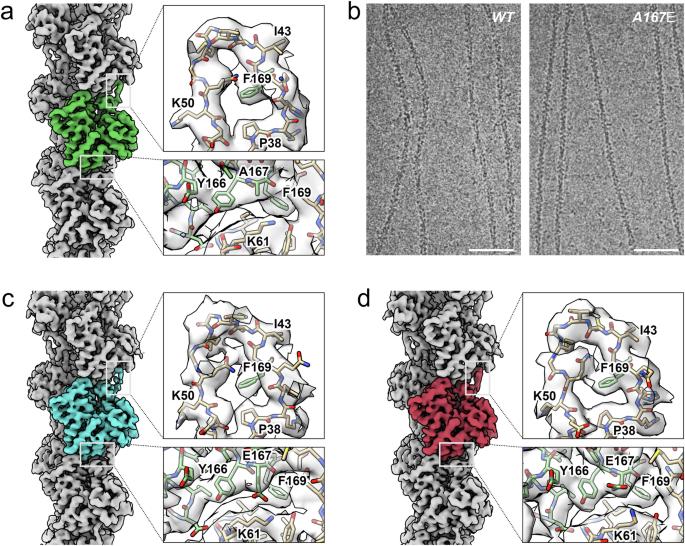
Actin filament assembly and the regulation of its mechanical properties are fundamental processes essential for eukaryotic cell function. Residue E167 in vertebrate actins forms an inter-subunit salt bridge with residue K61 of the adjacent subunit. Saccharomyces cerevisiae actin filaments are more flexible than vertebrate filaments and have an alanine at this position (A167). Substitution of this alanine for a glutamic acid (A167E) confers Saccharomyces cerevisiae actin filaments with salt-dependent stiffness similar to vertebrate actins. We developed an optimized cryogenic electron microscopy workflow refining sample preparation and vitrification to obtain near-atomic resolution structures of wild-type and A167E mutant Saccharomyces cerevisiae actin filaments. The difference between these structures allowed us to pinpoint the potential binding site of a filament-associated cation that controls the stiffness of the filaments in vertebrate and A167E Saccharomyces cerevisiae actins. Through an analysis of previously published high-resolution reconstructions of vertebrate actin filaments, along with a newly determined high-resolution vertebrate actin structure in the absence of potassium, we identified a unique peak near residue 167 consistent with the binding of a magnesium ion. Our findings show how magnesium can contribute to filament stiffening by directly bridging actin subunits and allosterically affecting the orientation of the DNase-I binding loop of actin, which plays a regulatory role in modulating actin filament stiffness and interactions with regulatory proteins. Actin filament assembly and the regulation of its mechanical properties are fundamental processes essential for eukaryotic cell function, however, the molecular mechanisms that govern the mechanical properties of the actin filaments formed from different species are not fully understood. Here, the authors report high-resolution cryo-EM reconstructions of yeast actin from Saccharomyces cerevisiae and propose how the mechanism of the stiffening of the actin filament is affected by magnesium cations.
高分辨率酵母肌动蛋白结构显示了阳离子使肌动蛋白丝变硬的分子机制。
肌动蛋白丝的组装及其机械特性的调节是真核细胞功能所必需的基本过程。脊椎动物肌动蛋白中的残基 E167 与相邻亚基的残基 K61 形成亚基间盐桥。酵母肌动蛋白丝比脊椎动物肌动蛋白丝更柔韧,在这个位置上有一个丙氨酸(A167)。将该丙氨酸替换为谷氨酸(A167E)可使酿酒酵母肌动蛋白丝具有与脊椎动物肌动蛋白相似的盐依赖性硬度。我们开发了一种优化的低温电子显微镜工作流程,改进了样品制备和玻璃化过程,从而获得了野生型和 A167E 突变体酿酒酵母肌动蛋白丝的近原子分辨率结构。根据这些结构之间的差异,我们确定了控制脊椎动物和 A167E 酿酒酵母肌动蛋白丝刚性的丝相关阳离子的潜在结合位点。通过分析以前发表的脊椎动物肌动蛋白丝的高分辨率重建图以及新测定的无钾条件下的高分辨率脊椎动物肌动蛋白结构,我们在残基 167 附近发现了一个独特的峰值,该峰值与镁离子的结合一致。我们的研究结果表明了镁如何通过直接桥接肌动蛋白亚基和异构影响肌动蛋白的 DNase-I 结合环的取向来促进肌动蛋白丝的僵化,而 DNase-I 结合环在调节肌动蛋白丝的僵化和与调节蛋白的相互作用方面起着调节作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


