PMN-MDSC: A Culprit Behind Immunosenescence and Increased Susceptibility to Clostridioides difficile Infection During Aging
IF 11.6
1区 工程技术
Q1 ENGINEERING, MULTIDISCIPLINARY
引用次数: 0
Abstract
Susceptibility to pathogens in the elderly is heightened with age, largely because of immunosenescence. As an immune regulatory organ, bone marrow creates immune cells that move to other organs and tissues through the blood. Despite the significance of this process of this organ, there is limited research on changes in immune cell generation in the bone marrow and their effects on immunosenescence. In this study, the compositions of immune cells in bone marrow from young (three months) and old (24+ months) mice were compared by means of mass cytometry, with further validation obtained through the reanalysis of single-cell RNA sequencing data and cell sorting via flow cytometry. The effects of differential immune cells on immunosenescence in old mice were evaluated using the Clostridium difficile (C. difficile) infection model. Our results showed that aged mice presented with a reduction in bone trabeculae structure, which was accompanied by a notable increase in polymorphonuclear (PMN)-myeloid-derived suppressor cell (MDSC) abundance. Through bulk-seq and reverse transcription quantitative polymerase chain reaction (RT-qPCR) analysis, we identified differential genes associated with the immune response—specifically, the Th17 cell differentiation pathway. Furthermore, the increase in exported PMN-MDSCs to the large intestine resulted in increased gut permeability and inflammatory damage to the colon following C. difficile infection. After clearing the PMN-MDSCs in old mice using the anti-Gr-1 antibody, the symptoms induced by C. difficile were significantly relieved, as evidenced by an inhibited IL-17 pathway in the colon and reduced gut permeability. In conclusion, aging increases the number of PMN-MDSCs in both the generated bone marrow and the outputted intestine, which contributes to susceptibility to C. difficile infection. This study provides a novel target for anti-aging therapy for immunosenescence, which is beneficial for improving immune function in elders.
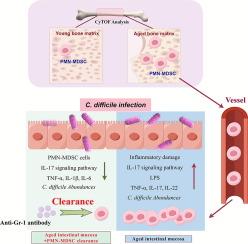
PMN-MDSC:衰老过程中免疫衰老和对艰难梭菌感染易感性增加的罪魁祸首
老年人对病原体的易感性随着年龄的增长而增加,这主要是由于免疫衰老所致。作为免疫调节器官,骨髓产生的免疫细胞通过血液转移到其他器官和组织。尽管该器官的这一过程非常重要,但有关骨髓中免疫细胞生成的变化及其对免疫衰老的影响的研究却很有限。在这项研究中,我们通过质谱细胞计数法比较了幼鼠(3 个月)和老 鼠(24 个月以上)骨髓中免疫细胞的组成,并通过重新分析单细胞 RNA 测序数据和流式细胞计数法进行细胞分拣进一步验证。利用艰难梭菌感染模型评估了差异免疫细胞对老年小鼠免疫衰老的影响。我们的研究结果表明,老年小鼠的骨小梁结构减少,同时多形核(PMN)-髓源性抑制细胞(MDSC)的数量明显增加。通过大量序列和反转录定量聚合酶链反应(RT-qPCR)分析,我们发现了与免疫反应相关的不同基因,特别是 Th17 细胞分化途径。此外,艰难梭菌感染后,向大肠输出的 PMN-MDSCs 增加导致肠道通透性增加和结肠炎症损伤。使用抗 Gr-1 抗体清除老龄小鼠的 PMN-MDSCs 后,艰难梭菌诱发的症状明显缓解,结肠中的 IL-17 通路受到抑制,肠道通透性降低就是证明。总之,衰老会增加生成的骨髓和输出的肠道中 PMN-MDSCs 的数量,从而导致对艰难梭菌感染的易感性。这项研究为免疫衰老的抗衰老疗法提供了一个新的靶点,有利于改善老年人的免疫功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Engineering
Environmental Science-Environmental Engineering
自引率
1.60%
发文量
335
审稿时长
35 days
期刊介绍:
Engineering, an international open-access journal initiated by the Chinese Academy of Engineering (CAE) in 2015, serves as a distinguished platform for disseminating cutting-edge advancements in engineering R&D, sharing major research outputs, and highlighting key achievements worldwide. The journal's objectives encompass reporting progress in engineering science, fostering discussions on hot topics, addressing areas of interest, challenges, and prospects in engineering development, while considering human and environmental well-being and ethics in engineering. It aims to inspire breakthroughs and innovations with profound economic and social significance, propelling them to advanced international standards and transforming them into a new productive force. Ultimately, this endeavor seeks to bring about positive changes globally, benefit humanity, and shape a new future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


