Ligand-mediated reactivity in CO oxidation of yttrium-nickel monoxide carbonyl complexes
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A series of heteronuclear yttrium-nickel monoxide carbonyl complexes YNiO(CO)n− (n = 1–5) were generated in a pulsed-laser vaporization source and characterized by mass-selected photoelectron velocity-map spectroscopy combined with theoretical calculations. CO ligand-mediated reactivity in CO oxidation of yttrium-nickel monoxide carbonyl complexes was experimentally and theoretically identified. During the consecutive CO adsorption, a μ2-O linear structure was most favorable for YNiO(CO)n− (n = 1, 2), then a structure in which the terminal O was bonded to the Y atom became favored for YNiO(CO)3−, and finally a structure bearing a CO2 moiety was most favorable for YNiO(CO)n− (n = 4, 5). Theoretical calculations indicated that the Ni atom acted as an electron acceptor and accumulated electron density at n ≤ 3, and then served as an electron donor along with the Y atom to contribute electron density in the rearrangement that accompanied CO oxidation at n > 3.
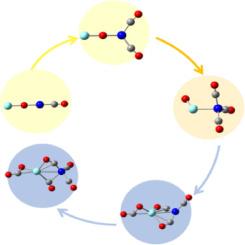
一氧化碳钇镍羰基复合物在 CO 氧化过程中配体介导的反应性
在脉冲激光气化源中生成了一系列异核钇镍一氧化碳羰基络合物 YNiO(CO)n-(n = 1-5),并通过质量选择光电子速度图谱结合理论计算对其进行了表征。实验和理论确定了一氧化碳钇镍羰基复合物在 CO 氧化过程中 CO 配体介导的反应性。在连续吸附 CO 的过程中,μ2-O 线性结构对 YNiO(CO)n- 最有利(n = 1、2),然后是末端 O 与 Y 原子成键的结构对 YNiO(CO)3- 最有利,最后是带有 CO2 分子的结构对 YNiO(CO)n- 最有利(n = 4、5)。理论计算表明,镍原子在 n ≤ 3 时充当电子受体并积累电子密度,然后与 Y 原子一起充当电子供体,在 n > 3 时伴随 CO 氧化的重排过程中贡献电子密度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


