Removal of contaminants from river Jakara using iron oxide nano particles prepared from Citrullus lanatus fruit waste
Abstract
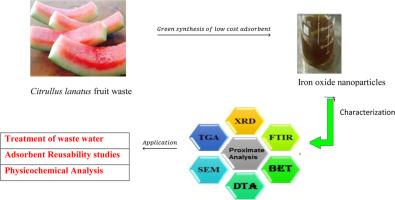
Achieving sustainable development requires efficient waste water treatment. Green synthesized iron nanoparticles have attracted much attention as potential catalysts for water remediation in view of their lost cost, high reactivity and good adsorption capacity. This study investigated the applicability of iron oxide nanoparticles synthesized from Citrullus lanatus fruit waste (IONP) in the remediation of contaminated water samples that were collected from River Jakara in Kano State Nigeria. The prepared nanoparticle was characterized using Brunauer–Emmett–Teller (BET) surface area, Scanning Electron Microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy and Thermogravimetric Analysis (TGA). The BET results revealed that IONP have large surface area and are nanometer sized particles. SEM analysis indicated that the adsorbent contain microsphere which might have facilitated the efficient purification of the river water while TGA study revealed that the adsorbent exhibited a three step decomposition process. Data obtained from XRD indicated that the synthesized adsorbent is of high purity and crystalline in nature with an average particle size of 17 nm. Results obtained after treatment of the river water with the adsorbent gave enhanced values of Total Dissolved Solids, Turbidity, Chemical Oxygen Demand, Dissolved Oxygen, phosphate and pH; thus confirming the high adsorption ability of the prepared nanoparticles. The percentage removal of Ni(II) Pb (II) and Cd (II) ions in the river water by IONP was found to depend on adsorbent concentration, agitation time and pH. The adsorption process of these metal ions onto the adsorbent was best described by the Langmuir isotherm model and followed pseudo second order kinetics. The regeneration stability of the adsorbent was adequate when treated with the heavy metals ions at optimum conditions. The nanoparticle synthesized from Citrullus lanatus waste was found to be an efficient and environmentally friendly alternative for treatment of contaminated water.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


