Co-transcriptional production of programmable RNA condensates and synthetic organelles
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
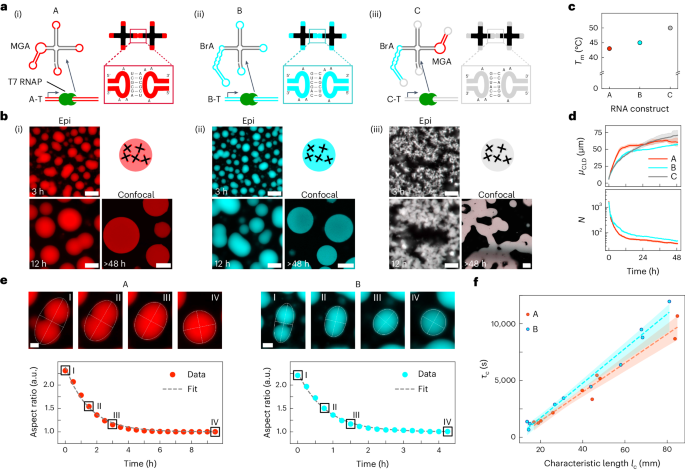
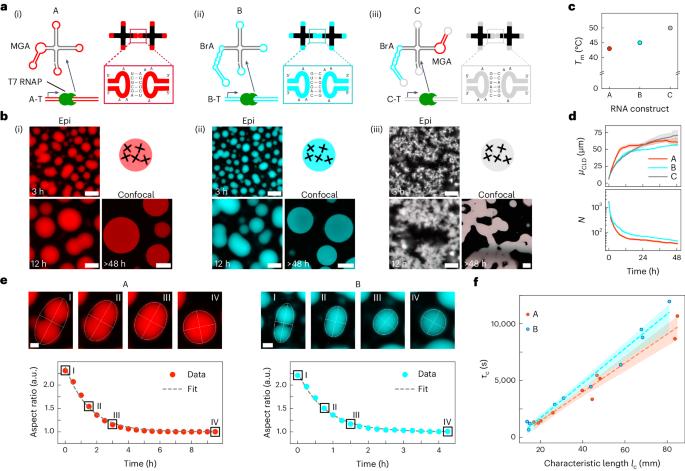
Condensation of RNA and proteins is central to cellular functions, and the ability to program it would be valuable in synthetic biology and synthetic cell science. Here we introduce a modular platform for engineering synthetic RNA condensates from tailor-made, branched RNA nanostructures that fold and assemble co-transcriptionally. Up to three orthogonal condensates can form simultaneously and selectively accumulate fluorophores through embedded fluorescent light-up aptamers. The RNA condensates can be expressed within synthetic cells to produce membrane-less organelles with a controlled number and relative size, and showing the ability to capture proteins using selective protein-binding aptamers. The affinity between otherwise orthogonal nanostructures can be modulated by introducing dedicated linker constructs, enabling the production of bi-phasic RNA condensates with a prescribed degree of interphase mixing and diverse morphologies. The in situ expression of programmable RNA condensates could underpin the spatial organization of functionalities in both biological and synthetic cells. Controlling RNA and protein condensation is helpful in synthetic biology. Here the authors show programmable assembly of synthetic RNA nanostructures into designer membrane-less organelles that selectively recruit ligands via protein-binding aptamers.
通过转录生产可编程 RNA 凝聚物和合成细胞器
RNA 和蛋白质的凝结是细胞功能的核心,对其进行编程的能力在合成生物学和合成细胞科学中非常重要。在这里,我们介绍了一个模块化平台,用于从量身定制的支化 RNA 纳米结构中设计合成 RNA 缩合物,这些 RNA 纳米结构可通过转录进行折叠和组装。最多可同时形成三个正交凝聚体,并通过嵌入的荧光发光适配体选择性地积聚荧光团。RNA 缩聚物可以在合成细胞中表达,产生数量和相对大小可控的无膜细胞器,并显示出利用选择性蛋白质结合适配体捕获蛋白质的能力。通过引入专用的连接构建物,可以调节原本正交的纳米结构之间的亲和力,从而生产出具有规定相间混合程度和不同形态的双相 RNA 凝聚体。可编程 RNA 凝聚物的原位表达可为生物细胞和合成细胞的功能空间组织提供支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


