mTORC2-driven chromatin cGAS mediates chemoresistance through epigenetic reprogramming in colorectal cancer
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
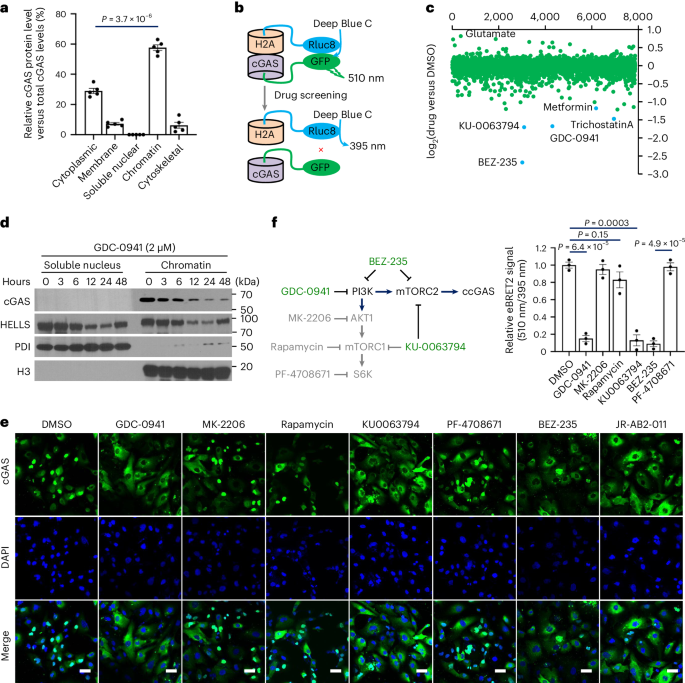
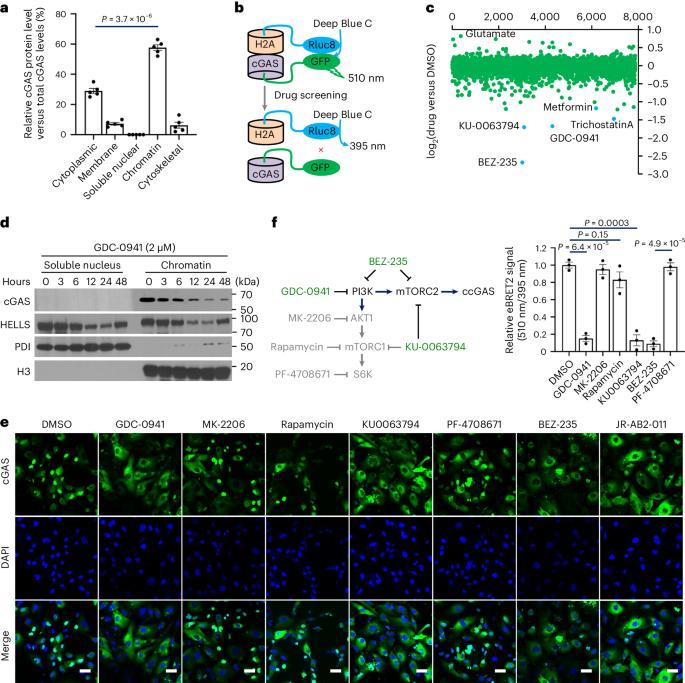
Cyclic GMP–AMP synthase (cGAS), a cytosolic DNA sensor that initiates a STING-dependent innate immune response, binds tightly to chromatin, where its catalytic activity is inhibited; however, mechanisms underlying cGAS recruitment to chromatin and functions of chromatin-bound cGAS (ccGAS) remain unclear. Here we show that mTORC2-mediated phosphorylation of human cGAS serine 37 promotes its chromatin localization in colorectal cancer cells, regulating cell growth and drug resistance independently of STING. We discovered that ccGAS recruits the SWI/SNF complex at specific chromatin regions, modifying expression of genes linked to glutaminolysis and DNA replication. Although ccGAS depletion inhibited cell growth, it induced chemoresistance to fluorouracil treatment in vitro and in vivo. Moreover, blocking kidney-type glutaminase, a downstream ccGAS target, overcame chemoresistance caused by ccGAS loss. Thus, ccGAS coordinates colorectal cancer plasticity and acquired chemoresistance through epigenetic patterning. Targeting both mTORC2–ccGAS and glutaminase provides a promising strategy to eliminate quiescent resistant cancer cells. Lv, Wang, Lin, Ye et al. report that mTORC2 phosphorylates cGAS to promote its chromatin localization and SWI/SNF recruitment to regulate target gene expression, thereby mediating plasticity and chemoresistance in colorectal cancer.
mTORC2 驱动的染色质 cGAS 通过表观遗传重编程介导结直肠癌的化疗抗药性
环状 GMP-AMP 合酶(cGAS)是一种细胞膜 DNA 传感器,可启动 STING 依赖性先天免疫反应,它可与染色质紧密结合,其催化活性在染色质中受到抑制;然而,cGAS 招募到染色质的机制以及与染色质结合的 cGAS(ccGAS)的功能仍不清楚。在这里,我们发现 mTORC2 介导的人 cGAS 丝氨酸 37 磷酸化促进了其在结直肠癌细胞中的染色质定位,从而独立于 STING 调节细胞生长和耐药性。我们发现 ccGAS 在特定染色质区域招募 SWI/SNF 复合物,从而改变与谷氨酰胺溶解和 DNA 复制相关的基因的表达。虽然 ccGAS 的缺失抑制了细胞的生长,但它在体外和体内诱导了对氟尿嘧啶治疗的化疗抗性。此外,阻断肾型谷氨酰胺酶(ccGAS的下游靶标)可克服ccGAS缺失导致的化疗抗性。因此,ccGAS通过表观遗传模式协调结直肠癌的可塑性和获得性化疗耐药性。同时以mTORC2-ccGAS和谷氨酰胺酶为靶点,为消除静止抗药性癌细胞提供了一种前景广阔的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


