Impact of the COVID-19 Pandemic on Conduct and Results of CLEAR Outcomes Trial
Abstract
Introduction
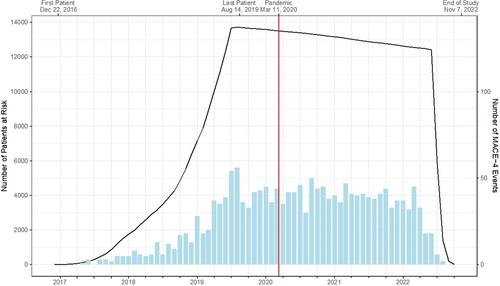
The COVID-19 pandemic disrupted clinical research. CLEAR Outcomes investigated the effect of bempedoic acid (BA) versus placebo in 13 970 patients with statin intolerance and high cardiovascular (CV) risk. BA reduced the risk of the primary endpoint (composite of CV death, nonfatal myocardial infarction, nonfatal stroke, or coronary revascularization) by 13%. CLEAR Outcomes began before and continued for 2.7 years after the start of the pandemic.
Methods
The impact of the COVID-19 pandemic on patient disposition, adverse events, and major adverse CV events (MACE) in CLEAR Outcomes was assessed.
Results
Rates of severe infection, hospitalization, or first MACE associated with a positive COVID-19 test were low and balanced between treatment groups. Rates of all-cause death, non-CV death, and undetermined death increased in the pandemic period compared with the pre-pandemic period, while rates of CV death with a known etiology remained stable. A sensitivity analysis excluding undetermined deaths occurring after the onset of the pandemic from the CV death designation yielded hazard ratios of 0.84 (95% CI, 0.76–0.93) for the primary endpoint and 0.94 (95% CI, 0.76–1.16) for the secondary endpoint of CV death, compared with 0.87 (95% CI, 0.79–0.96) and 1.04 (95% CI, 0.88–1.24), respectively, in the original analysis.
Conclusion
The CLEAR Outcomes trial continued uninterrupted throughout the COVID-19 pandemic. Certain trial endpoints may have been impacted by the pandemic. Specifically, the classification of undetermined deaths as CV deaths may have attenuated the effect of BA on key efficacy endpoints.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


