An Automated Purification Workflow Coupled with Material-Sparing High-Throughput 1H NMR for Parallel Medicinal Chemistry
IF 3.5
3区 医学
Q2 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
In medicinal chemistry, purification and characterization of organic compounds is an ever-growing challenge, with an increasing number of compounds being synthesized at a decreased scale of preparation. In response to this trend, we developed a parallel medicinal chemistry (PMC)-tailored platform, coupling automated purification to mass spectrometry (MS) and nuclear magnetic resonance spectroscopy (NMR) on a range of synthetic scales (∼3.0–75.0 μmol). Here, the generation and acquisition of 1.7 mm NMR samples is fully integrated into a high-throughput automated workflow, processing 36 000 compounds yearly. Utilizing dead volume, which is inaccessible in conventional liquid handling, NMR samples are generated on as little as 10 μg without consuming material prioritized for biological assays. As miniaturized PMC synthesis becomes the industry standard, we can now obtain quality NMR spectra from limited material. Paired with automated structure verification, this platform has the potential to allow NMR to become as important for high-throughput analysis as ultrahigh performance liquid chromatography (UPLC)-MS.
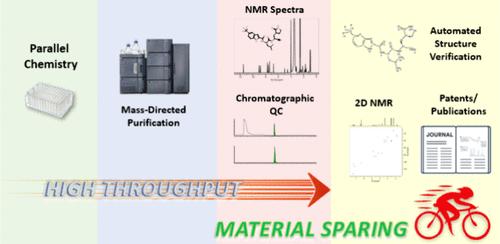
用于平行药物化学的自动纯化工作流程与节省材料的高通量 1H NMR 相结合
在药物化学领域,有机化合物的纯化和表征是一项日益严峻的挑战,因为越来越多的化合物是在制备规模缩小的情况下合成的。针对这一趋势,我们开发了一个平行药物化学(PMC)定制平台,在一系列合成规模(∼3.0-75.0 μmol)上将自动纯化与质谱(MS)和核磁共振光谱(NMR)结合起来。在这里,1.7 毫米 NMR 样品的生成和采集完全集成到一个高通量自动化工作流程中,每年可处理 36 000 个化合物。利用传统液体处理无法进入的死体积,只需 10 μg 就能生成 NMR 样品,而不会消耗优先用于生物检测的材料。随着小型化 PMC 合成成为行业标准,我们现在可以从有限的材料中获得高质量的 NMR 图谱。配合自动结构验证,该平台有望使 NMR 成为与超高效液相色谱 (UPLC)-MS 一样重要的高通量分析工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
ACS Medicinal Chemistry Letters
CHEMISTRY, MEDICINAL-
CiteScore
7.30
自引率
2.40%
发文量
328
审稿时长
1 months
期刊介绍:
ACS Medicinal Chemistry Letters is interested in receiving manuscripts that discuss various aspects of medicinal chemistry. The journal will publish studies that pertain to a broad range of subject matter, including compound design and optimization, biological evaluation, drug delivery, imaging agents, and pharmacology of both small and large bioactive molecules. Specific areas include but are not limited to:
Identification, synthesis, and optimization of lead biologically active molecules and drugs (small molecules and biologics)
Biological characterization of new molecular entities in the context of drug discovery
Computational, cheminformatics, and structural studies for the identification or SAR analysis of bioactive molecules, ligands and their targets, etc.
Novel and improved methodologies, including radiation biochemistry, with broad application to medicinal chemistry
Discovery technologies for biologically active molecules from both synthetic and natural (plant and other) sources
Pharmacokinetic/pharmacodynamic studies that address mechanisms underlying drug disposition and response
Pharmacogenetic and pharmacogenomic studies used to enhance drug design and the translation of medicinal chemistry into the clinic
Mechanistic drug metabolism and regulation of metabolic enzyme gene expression
Chemistry patents relevant to the medicinal chemistry field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


