Exploring Advanced CRISPR Delivery Technologies for Therapeutic Genome Editing
IF 11.1
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
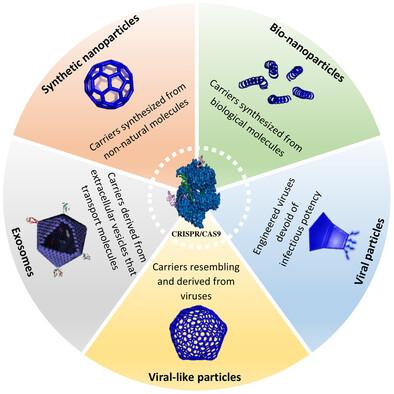
The genetic material within cells plays a pivotal role in shaping the structure and function of living organisms. Manipulating an organism's genome to correct inherited abnormalities or introduce new traits holds great promise. Genetic engineering techniques offers promising pathways for precisely altering cellular genetics. Among these methodologies, clustered regularly interspaced short palindromic repeat (CRISPR), honored with the 2020 Nobel Prize in Chemistry, has garnered significant attention for its precision in editing genomes. However, the CRISPR system faces challenges when applied in vivo, including low delivery efficiency, off-target effects, and instability. To address these challenges, innovative technologies for targeted and precise delivery of CRISPR have emerged. Engineered carrier platforms represent a substantial advancement, improving stability, precision, and reducing the side effects associated with genome editing. These platforms facilitate efficient local and systemic genome engineering of various tissues and cells, including immune cells. This review explores recent advances, benefits, and challenges of CRISPR-based genome editing delivery. It examines various carriers including nanocarriers (polymeric, lipid-derived, metallic, and bionanoparticles), viral particles, virus-like particles, and exosomes, providing insights into their clinical utility and future prospects.
探索用于治疗性基因组编辑的先进 CRISPR 传输技术
细胞内的遗传物质在塑造生物体的结构和功能方面起着关键作用。操纵生物体的基因组来纠正遗传异常或引入新的性状大有可为。基因工程技术为精确改变细胞遗传学提供了前景广阔的途径。在这些方法中,荣获 2020 年诺贝尔化学奖的聚类规则间隔短回文重复(CRISPR)因其在编辑基因组方面的精确性而备受关注。然而,CRISPR 系统在体内应用时面临着各种挑战,包括传递效率低、脱靶效应和不稳定性。为了应对这些挑战,CRISPR 的靶向精准递送创新技术应运而生。工程载体平台是一项重大进步,它提高了稳定性和精确性,并减少了与基因组编辑相关的副作用。这些平台有助于对各种组织和细胞(包括免疫细胞)进行高效的局部和全身基因组工程。本综述探讨了基于 CRISPR 的基因组编辑传递的最新进展、优势和挑战。它研究了各种载体,包括纳米载体(聚合物、脂源、金属和仿生颗粒)、病毒颗粒、类病毒颗粒和外泌体,深入探讨了它们的临床实用性和未来前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
14.00
自引率
2.40%
发文量
0
期刊介绍:
Small Science is a premium multidisciplinary open access journal dedicated to publishing impactful research from all areas of nanoscience and nanotechnology. It features interdisciplinary original research and focused review articles on relevant topics. The journal covers design, characterization, mechanism, technology, and application of micro-/nanoscale structures and systems in various fields including physics, chemistry, materials science, engineering, environmental science, life science, biology, and medicine. It welcomes innovative interdisciplinary research and its readership includes professionals from academia and industry in fields such as chemistry, physics, materials science, biology, engineering, and environmental and analytical science. Small Science is indexed and abstracted in CAS, DOAJ, Clarivate Analytics, ProQuest Central, Publicly Available Content Database, Science Database, SCOPUS, and Web of Science.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


