Sustainable Spinning of Artificial Spider Silk Fibers with Excellent Toughness and Inherent Potential for Functionalization
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
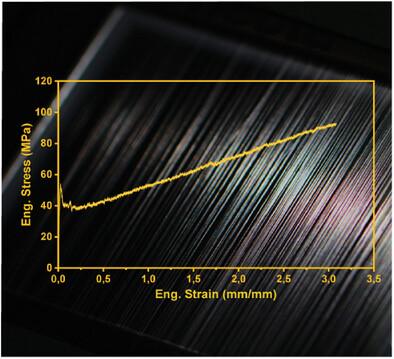
Despite impressive progress in the field, there are still several major bottlenecks in producing fibers from recombinantly produced spider‐silk‐like proteins to replicate the extraordinary mechanical properties of spider major ampullate silk. The conventional artificial fiber spinning processes rely primarily on organic solvents to coagulate proteins into fibers and require complex post‐treatments to obtain fibers with valuable properties. This is due to challenges in obtaining soluble silk proteins, but also because the native silk spinning process leading to the hierarchical organization of the silk proteins is not fully understood and is hard to replicate in a manner applicable to industrial settings. Here, recombinant spider‐silk fusion proteins are efficiently produced and processed into as‐spun fibers with a toughness modulus of 120 MJ m
可持续纺制具有优异韧性和固有功能化潜力的人造蜘蛛丝纤维
尽管该领域取得了令人瞩目的进展,但在利用重组生产的类蜘蛛丝蛋白生产纤维以复制蜘蛛主要安培蚕丝的非凡机械特性方面,仍存在几个主要瓶颈。传统的人造纤维纺丝工艺主要依靠有机溶剂将蛋白质凝结成纤维,并需要复杂的后处理才能获得具有重要特性的纤维。这是由于在获得可溶性蚕丝蛋白方面存在挑战,同时也是因为导致蚕丝蛋白分层组织的原生蚕丝纺丝过程尚未完全被理解,并且难以以适用于工业环境的方式进行复制。在这里,仅使用水溶液就能高效生产重组蜘蛛丝融合蛋白,并将其加工成韧性模量为 120 MJ m-3 和延展性为 255% 的原丝纤维。蛛丝融合蛋白的组装方式与已报道的原生蛛丝的组装方式类似:它们在盐析作用下发生相分离,然后在剪切力和脱水作用下发生排列和二级结构转变。最后,融合蚕丝蛋白的设计使得在温和的全水条件下,通过简单的生物分子点击反应,在纺丝前和纺丝后对纤维进行直接的功能化处理成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


