Protein Complex Heterogeneity and Topology Revealed by Electron Capture Charge Reduction and Surface Induced Dissociation
IF 12.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
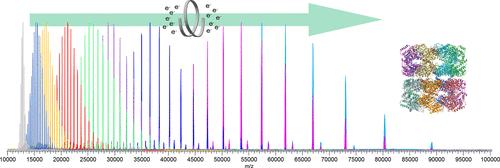
We illustrate the utility of native mass spectrometry (nMS) combined with a fast, tunable gas-phase charge reduction, electron capture charge reduction (ECCR), for the characterization of protein complex topology and glycoprotein heterogeneity. ECCR efficiently reduces the charge states of tetradecameric GroEL, illustrating Orbitrap m/z measurements to greater than 100,000 m/z. For pentameric C-reactive protein and tetradecameric GroEL, our novel device combining ECCR with surface induced dissociation (SID) reduces the charge states and yields more topologically informative fragmentation. This is the first demonstration that ECCR yields more native-like SID fragmentation. ECCR also significantly improved mass and glycan heterogeneity measurements of heavily glycosylated SARS-CoV-2 spike protein trimer and thyroglobulin dimer. Protein glycosylation is important for structural and functional properties and plays essential roles in many biological processes. The immense heterogeneity in glycosylation sites and glycan structure poses significant analytical challenges that hinder a mechanistic understanding of the biological role of glycosylation. Without ECCR, average mass determination of glycoprotein complexes is available only through charge detection mass spectrometry or mass photometry. With narrow m/z selection windows followed by ECCR, multiple glycoform m/z values are apparent, providing quick global glycoform profiling and providing a future path for glycan localization on individual intact glycoforms.
通过电子捕获电荷还原和表面诱导解离揭示蛋白质复合物的异质性和拓扑结构
我们展示了本征质谱(nMS)与快速可调气相电荷还原法(电子捕获电荷还原法,ECCR)相结合在表征蛋白质复合物拓扑结构和糖蛋白异质性方面的实用性。电子捕获电荷还原可有效还原十四聚体 GroEL 的电荷状态,Orbitrap m/z 测量值大于 100,000 m/z。对于五聚体 C 反应蛋白和十四聚体 GroEL,我们的新型装置将 ECCR 与表面诱导解离 (SID) 结合在一起,减少了电荷状态,产生了拓扑信息更丰富的碎片。这是首次证明 ECCR 能产生更像原生的 SID 片段。ECCR 还大大改善了重度糖基化的 SARS-CoV-2 穗状蛋白三聚体和甲状腺球蛋白二聚体的质量和糖异质性测量。蛋白质糖基化对其结构和功能特性非常重要,在许多生物过程中发挥着至关重要的作用。糖基化位点和聚糖结构的巨大异质性给分析带来了巨大挑战,阻碍了对糖基化生物学作用的机理理解。没有 ECCR,糖蛋白复合物的平均质量测定只能通过电荷检测质谱法或质量光度法进行。通过 ECCR 后的窄 m/z 选择窗口,多个糖基化形式的 m/z 值就会显现出来,从而提供快速的全局糖基化形式剖析,并为今后对单个完整糖基化形式进行聚糖定位提供了途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


