Dissecting the neuroprotective interaction between the BH4 domain of BCL-w and the IP3 receptor
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
BCL-w is a BCL-2 family protein that promotes cell survival in tissue- and disease-specific contexts. The canonical anti-apoptotic functionality of BCL-w is mediated by a surface groove that traps the BCL-2 homology 3 (BH3) α-helices of pro-apoptotic members, blocking cell death. A distinct N-terminal portion of BCL-w, termed the BCL-2 homology 4 (BH4) domain, selectively protects axons from paclitaxel-induced degeneration by modulating IP3 receptors, a noncanonical BCL-2 family target. Given the potential of BCL-w BH4 mimetics to prevent or mitigate chemotherapy-induced peripheral neuropathy, we sought to characterize the interaction between BCL-w BH4 and the IP3 receptor, combining “staple” and alanine scanning approaches with molecular dynamics simulations. We generated and identified stapled BCL-w BH4 peptides with optimized IP3 receptor binding and neuroprotective activities. Point mutagenesis further revealed the sequence determinants for BCL-w BH4 specificity, providing a blueprint for therapeutic targeting of IP3 receptors to achieve neuroprotection.
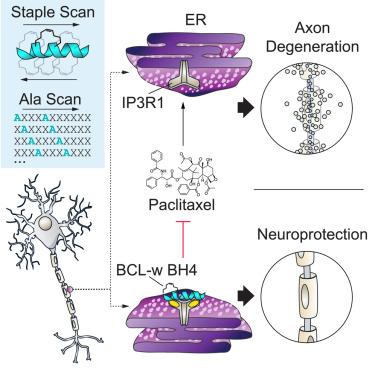
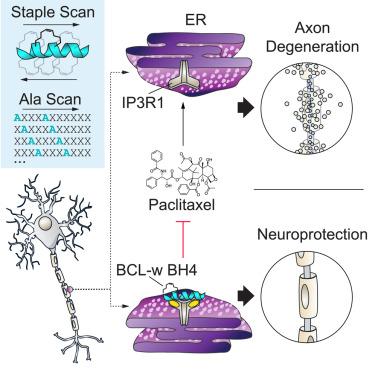
剖析 BCL-w 的 BH4 结构域与 IP3 受体之间的神经保护相互作用
BCL-w是一种BCL-2家族蛋白,可在组织和疾病特异性环境中促进细胞存活。BCL-w 的典型抗凋亡功能由一个表面沟槽介导,该沟槽能捕获促凋亡成员的 BCL-2 同源 3 (BH3) α-螺旋,从而阻止细胞死亡。BCL-w的一个独特的N端部分被称为BCL-2同源4(BH4)结构域,它通过调节IP3受体(BCL-2家族的一个非经典靶点),选择性地保护轴突免受紫杉醇诱导的变性。鉴于 BCL-w BH4 拟定物有可能预防或减轻化疗诱导的周围神经病变,我们将 "钉书针 "和丙氨酸扫描方法与分子动力学模拟相结合,试图描述 BCL-w BH4 与 IP3 受体之间的相互作用。我们生成并鉴定了具有优化的 IP3 受体结合和神经保护活性的订书钉 BCL-w BH4 肽。点突变进一步揭示了 BCL-w BH4 特异性的序列决定因素,为靶向 IP3 受体实现神经保护提供了治疗蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


