Zn-Cu bimetallic gas diffusion electrodes for electrochemical reduction of CO2 to ethylene
Abstract
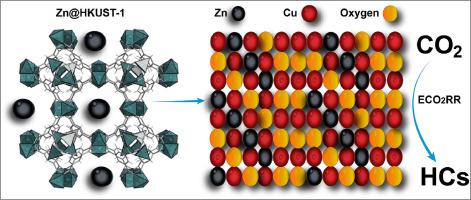
The application of renewable energy to drive the electroreduction of carbon dioxide (CO2) into valuable compounds serves as a means of energy storage and simultaneously aids in the mitigation of climate change. In this study, a bimetallic catalyst consisting of Zinc and Copper is synthesized using the Zinc-doped HKUST-1 metal-organic framework. The catalyst is then fabricated on gas diffusional electrode and investigated in both H-Cell and Flow cell configurations for electrochemical CO2 reduction reaction (ECO2RR). In the H-Cell, the catalysts that have been developed exhibit an ethylene faradaic efficiency (FE) up to 40 % when operated at a potential of −0.8 V relative to the reversible hydrogen electrode (RHE). The Zn-Cu bimetallic gas diffusion electrodes (GDE) exhibit an impressive ethylene FE up to 45 % when operating at a current density of −200 mA cm−2 at −1.0 V versus RHE in the flow cell. This research offers valuable insights into the strategic development of copper-based bimetallic catalysts with the aim of enhancing the efficiency of electrochemical reduction of CO2 to yield multicarbon compounds.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


