Safety and tolerability of the protein C activator AB002 in end-stage renal disease patients on hemodialysis: a randomized phase 2 trial
IF 5.4
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
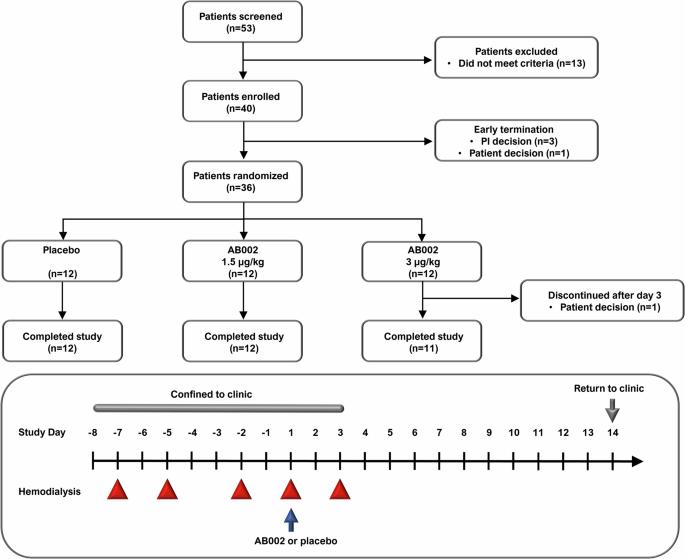
The protein C system regulates blood coagulation, inflammation, and vascular integrity. AB002 is an injectable protein C activating enzyme under investigation to safely prevent and treat thrombosis. In preclinical models, AB002 is antithrombotic, cytoprotective, and anti-inflammatory. Since prophylactic use of heparin is contraindicated during hemodialysis in some end-stage renal disease (ESRD) patients, we propose using AB002 as a short-acting alternative to safely limit blood loss due to clotting in the dialysis circuit. This phase 2, randomized, double-blind, placebo-controlled, single-dose study evaluates the safety and tolerability of AB002 administered into the hemodialysis line of ESRD patients during hemodialysis at one study center in the United States (ClinicalTrials.gov: NCT03963895). In this study, 36 patients were sequentially enrolled into two cohorts and randomized to AB002 or placebo in a 2:1 ratio. In cohort 1, patients received 1.5 µg/kg AB002 (n = 12) or placebo (n = 6); in cohort 2, patients received 3 µg/kg AB002 (n = 12) or placebo (n = 6). Patients underwent five heparin-free hemodialysis sessions over 10 days and were dosed with AB002 or placebo during session four. Here we show that AB002 is safe and well-tolerated in ESRD patients, with no treatment-related adverse events. Clinically relevant bleeding did not occur in any patient, and the time to hemostasis at the vascular access sites is not affected by AB002. As far as we are aware, this proof-of-concept study is the first clinical trial assessing the therapeutic potential of protein C activation. The results herein support additional investigation of AB002 to safely prevent and treat thrombosis in at-risk populations. Some people with kidney disease require hemodialysis, a process in which a machine filters the blood to remove waste products. The process of hemodialysis can trigger blood clotting in the hemodialysis circuit. Therefore, the blood-thinner heparin is commonly used to prevent blood from clotting. However, some patients cannot tolerate heparin. Here we describe a clinical trial in which we tested whether a drug called AB002 is safe and can reduce hemodialysis circuit clotting in people with permanent kidney disease (end-stage renal disease) undergoing hemodialysis. AB002 appears to be safe and well-tolerated, and we observed reduced clotting without any signs of increased bleeding. Further studies are required in more patients to determine whether AB002 can be used routinely during hemodialysis to safely prevent or treat blood clots. Verbout et al. report findings from a phase 2 proof-of-concept study of the protein C activator AB002 in patients with end stage renal disease undergoing heparin-free hemodialysis. AB002 appears safe and well-tolerated, while demonstrating reduced dialyzer clot severity and no increased bleeding at the vascular access site compared to placebo.
血液透析终末期肾病患者使用蛋白 C 激活剂 AB002 的安全性和耐受性:随机 2 期试验。
背景:蛋白 C 系统调节血液凝固、炎症和血管完整性。AB002 是一种可注射的蛋白 C 激活酶,目前正在研究如何安全地预防和治疗血栓形成。在临床前模型中,AB002 具有抗血栓、细胞保护和抗炎作用。由于一些终末期肾病(ESRD)患者在血液透析期间禁忌预防性使用肝素,因此我们建议使用 AB002 作为短效替代品,以安全地限制透析回路中因凝血造成的失血量:这项 2 期随机、双盲、安慰剂对照、单剂量研究评估了美国一家研究中心在 ESRD 患者血液透析期间在血液透析管路中注射 AB002 的安全性和耐受性(ClinicalTrials.gov:NCT03963895)。在这项研究中,36 名患者按顺序被纳入两个队列,并以 2:1 的比例随机接受 AB002 或安慰剂治疗。第一组患者接受 1.5 µg/kg AB002(12 人)或安慰剂(6 人)治疗;第二组患者接受 3 µg/kg AB002(12 人)或安慰剂(6 人)治疗。患者在10天内进行了5次不含肝素的血液透析,并在第4次透析期间服用了AB002或安慰剂:结果:我们在此表明,AB002 在 ESRD 患者中安全且耐受性良好,未出现与治疗相关的不良事件。所有患者均未发生临床相关出血,血管通路部位的止血时间不受 AB002 的影响:据我们所知,这项概念验证研究是首个评估蛋白 C 激活治疗潜力的临床试验。本文的结果支持对 AB002 进行更多研究,以安全地预防和治疗高危人群的血栓形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


