Phospholipid biosynthesis modulates nucleotide metabolism and reductive capacity
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Phospholipid and nucleotide syntheses are fundamental metabolic processes in eukaryotic organisms, with their dysregulation implicated in various disease states. Despite their importance, the interplay between these pathways remains poorly understood. Using genetic and metabolic analyses in Saccharomyces cerevisiae, we elucidate how cytidine triphosphate usage in the Kennedy pathway for phospholipid synthesis influences nucleotide metabolism and redox balance. We find that deficiencies in the Kennedy pathway limit nucleotide salvage, prompting compensatory activation of de novo nucleotide synthesis and the pentose phosphate pathway. This metabolic shift enhances the production of antioxidants such as NADPH and glutathione. Moreover, we observe that the Kennedy pathway for phospholipid synthesis is inhibited during replicative aging, indicating its role in antioxidative defense as an adaptive mechanism in aged cells. Our findings highlight the critical role of phospholipid synthesis pathway choice in the integrative regulation of nucleotide metabolism, redox balance and membrane properties for cellular defense. Zhu et al. found that cytidine triphosphate usage in the Kennedy pathway for phospholipid synthesis influences nucleotide metabolism and redox balance. Phospholipid synthesis acts as an integrative defense mechanism to sense and combat oxidative stress.
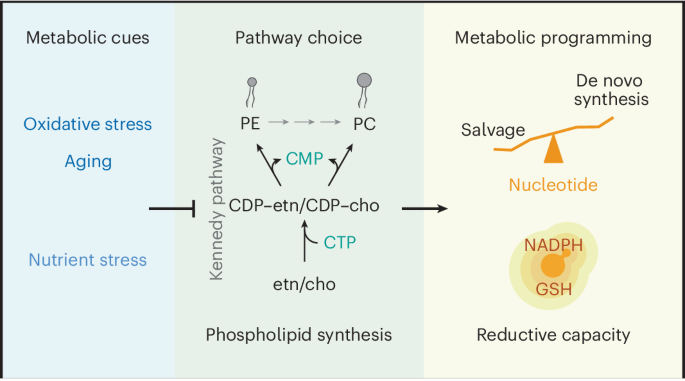

磷脂生物合成调节核苷酸代谢和还原能力
磷脂和核苷酸合成是真核生物的基本代谢过程,它们的失调与各种疾病状态有关。尽管它们非常重要,但人们对这些途径之间的相互作用仍然知之甚少。通过对酿酒酵母的遗传和代谢分析,我们阐明了三磷酸胞苷在肯尼迪磷脂合成途径中的使用如何影响核苷酸代谢和氧化还原平衡。我们发现,肯尼迪途径的缺陷限制了核苷酸的挽救,促使核苷酸的从头合成和磷酸戊糖途径的补偿性激活。这种新陈代谢的转变增强了 NADPH 和谷胱甘肽等抗氧化剂的生成。此外,我们观察到肯尼迪磷脂合成途径在复制衰老过程中受到抑制,这表明它在抗氧化防御中的作用是衰老细胞的一种适应机制。我们的发现凸显了磷脂合成途径选择在核苷酸代谢、氧化还原平衡和细胞膜特性的综合调控中对细胞防御的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


