In vivo monitoring of active subretinal fibrosis in mice using collagen hybridizing peptides
IF 5.9
3区 农林科学
Q1 VETERINARY SCIENCES
引用次数: 0
Abstract
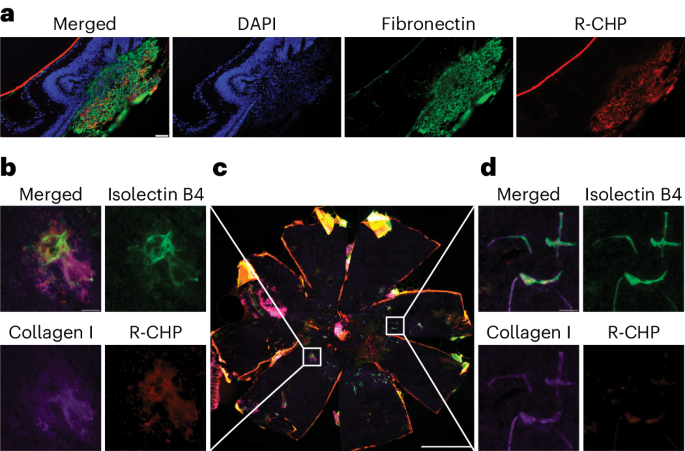
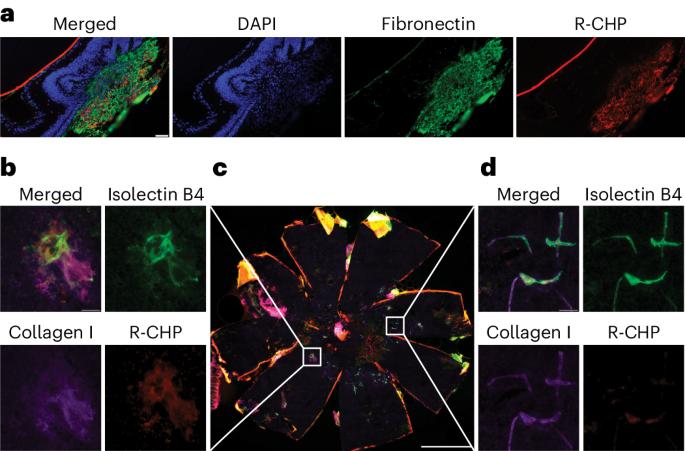
Subretinal fibrosis is associated with worse visual outcomes in patients with neovascular age-related macular degeneration. As there is a lack of optimal biomarkers and no method that directly detects collagen in the back of the eye, novel tools that monitor fibrosis-related changes in neovascular age-related macular degeneration are needed. Here, using two mouse models (the laser-induced choroidal neovascularization model, and the JR5558 mouse presenting with spontaneous subretinal neovascularization with fibrosis), we imaged active fibrotic lesions using fluorescently labeled collagen hybridizing peptides (CHPs), short peptides that bind to single α-chain collagen structures during collagen remodeling. JR5558 retinal pigment epithelium/choroid flat mounts showed CHP co-staining with fibrosis and epithelial mesenchymal transition-related markers; additionally, CHP histopathology staining correlated with in vivo CHP imaging. After laser-induced choroidal neovascularization, in vivo CHP binding correlated with laser intensity, histopathology CHP and fibronectin staining. Laser-induced choroidal neovascularization showed decreased CHP intensity over time in healing/regressing versus active scars in vivo, whereas increased CHP binding correlated with elevated fibrosis in JR5558 mouse eyes with age. In bispecific angiopoietin 2/vascular endothelial growth factor antibody-treated JR5558 mice, CHPs detected significantly decreased collagen remodeling versus immunoglobulin G control. These results demonstrate the first use of CHPs to directly image remodeling collagen in the eye and as a potential clinical optical biomarker of active subretinal fibrosis associated with ocular neovascularization. This study reports the use of fluorescently labeled collagen hybridizing peptides to directly image collagen remodeling and monitor fibrosis in two mouse models of ocular neovascularization.
利用胶原杂交肽对小鼠视网膜下活动性纤维化进行体内监测
视网膜下纤维化与新生血管性老年黄斑变性患者视力下降有关。由于缺乏最佳生物标志物,也没有直接检测眼底胶原蛋白的方法,因此需要新型工具来监测新生血管性老年黄斑变性中与纤维化相关的变化。在这里,我们利用两种小鼠模型(激光诱导的脉络膜新生血管模型和自发性视网膜下新生血管伴纤维化的 JR5558 小鼠),使用荧光标记的胶原杂交肽(CHPs)对活跃的纤维化病变进行成像,CHPs 是在胶原重塑过程中与单个 α 链胶原结构结合的短肽。JR5558 视网膜色素上皮/脉络膜平片显示 CHP 与纤维化和上皮间质转化相关标记物共同染色;此外,CHP 组织病理学染色与体内 CHP 成像相关。激光诱导脉络膜新生血管后,体内 CHP 结合与激光强度、组织病理学 CHP 和纤维连接蛋白染色相关。激光诱导的脉络膜新生血管在体内愈合/退行性疤痕与活动性疤痕中的 CHP 强度随时间推移而降低,而 CHP 结合力的增加与 JR5558 小鼠眼球纤维化程度随年龄增长而升高有关。在双特异性血管生成素 2/血管内皮生长因子抗体处理的 JR5558 小鼠中,CHPs 检测到的胶原重塑与免疫球蛋白 G 对照组相比明显减少。这些结果表明,CHPs 首次用于直接成像眼球中重塑的胶原蛋白,并可作为一种潜在的临床光学生物标记,用于检测与眼球新生血管相关的活动性视网膜下纤维化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Lab Animal
农林科学-兽医学
CiteScore
0.60
自引率
2.90%
发文量
181
审稿时长
>36 weeks
期刊介绍:
LabAnimal is a Nature Research journal dedicated to in vivo science and technology that improves our basic understanding and use of model organisms of human health and disease. In addition to basic research, methods and technologies, LabAnimal also covers important news, business and regulatory matters that impact the development and application of model organisms for preclinical research.
LabAnimal's focus is on innovative in vivo methods, research and technology covering a wide range of model organisms. Our broad scope ensures that the work we publish reaches the widest possible audience. LabAnimal provides a rigorous and fair peer review of manuscripts, high standards for copyediting and production, and efficient publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


