“Two-birds-one-stone” oral nanotherapeutic designed to target intestinal integrins and regulate redox homeostasis for UC treatment
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
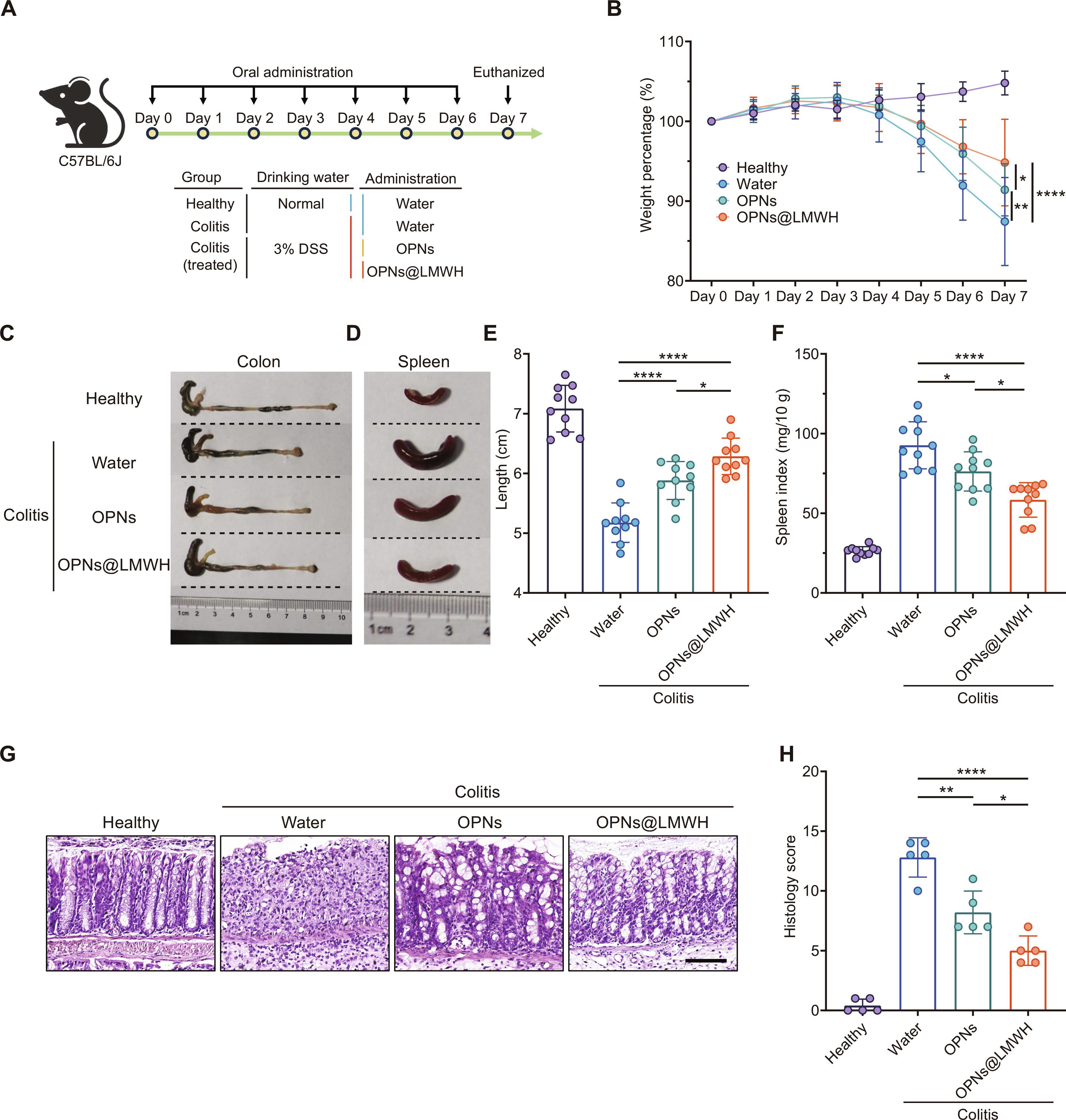
Designing highly efficient orally administrated nanotherapeutics with specific inflammatory site–targeting functions in the gastrointestinal tract for ulcerative colitis (UC) management is a noteworthy challenge. Here, we focused on exploring a specific targeting oral nanotherapy, serving as “one stone,” for the directed localization of inflammation and the regulation of redox homeostasis, thereby achieving effects against “two birds” for UC treatment. Our designed nanotherapeutic agent OPNs@LMWH (oxidation-sensitive ε-polylysine nanoparticles at low–molecular weight heparin) exhibited specific active targeting effects and therapeutic efficacy simultaneously. Our results indicate that OPNs@LMWH had high integrin αM–mediated immune cellular uptake efficiency and preferentially accumulated in inflamed tissues. We also confirmed its effectiveness in the treatment experiment of colitis in mice by ameliorating oxidative stress and inhibiting the activation of inflammation-associated signaling pathways while simultaneously bolstering the protective mechanisms of the colonic epithelium. Overall, these findings underscore the compelling dual functionalities of OPNs@LMWH, which enable effective oral delivery to inflamed sites, thereby facilitating precise UC management.
"双鸟一石 "口服纳米疗法,旨在靶向肠道整合素和调节氧化还原稳态,治疗多发性硬化症。
设计具有胃肠道特定炎症位点靶向功能的高效口服纳米疗法来治疗溃疡性结肠炎(UC)是一项值得关注的挑战。在此,我们重点探索一种特异性靶向口服纳米疗法,作为 "一石",定向定位炎症和调节氧化还原平衡,从而达到治疗溃疡性结肠炎的 "一箭双雕 "效果。我们设计的纳米治疗剂OPNs@LMWH(低分子量肝素的氧化敏感性ε-聚赖氨酸纳米颗粒)同时表现出特异性的活性靶向效应和治疗效果。我们的研究结果表明,OPNs@LMWH 具有较高的整合素 αM 介导的免疫细胞摄取效率,并优先在炎症组织中积累。我们还证实了它在小鼠结肠炎治疗实验中的有效性,它能改善氧化应激,抑制炎症相关信号通路的激活,同时增强结肠上皮的保护机制。总之,这些研究结果强调了 OPNs@LMWH 令人信服的双重功能,它能有效地通过口服输送到发炎部位,从而促进对 UC 的精确管理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


