Oral administration of garlic-derived nanoparticles improves cancer immunotherapy by inducing intestinal IFNγ-producing γδ T cells
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Gamma-delta (γδ) T cell-based cancer immunotherapies represent a promising avenue for cancer treatment. However, their development is challenged by the limited expansion and differentiation of the cells ex vivo. Here we induced the endogenous expansion and activation of γδ T cells through oral administration of garlic-derived nanoparticles (GNPs). We found that GNPs could significantly promote the proliferation and activation of endogenous γδ T cells in the intestine, leading to generation of large amount of interferon-γ (IFNγ). Moreover GNP-treated mice showed increased levels of chemokine CXCR3 in intestinal γδ T cells, which can drive their migration from the gut to the tumour environment. The translocation of γδ T cells and IFNγ from the intestine to extraintestinal subcutaneous tumours remodels the tumour immune microenvironment and synergizes with anti-PD-L1, inducing robust antitumour immunity. Our study delineates mechanistic insight into the complex gut–tumour interactome and provides an alternative approach for γδ T cell-based immunotherapy. γδ T cell-based immunotherapies are limited by the reduced expansion and activation of the T cells ex vivo. Here the authors show that garlic nanoparticles can activate IFNγ-producing γδ T cells directly in the intestine, before they translocate to extraintestinal tumours, where they can synergize with anti-PD-L1 therapy, inducing robust antitumour immunity.
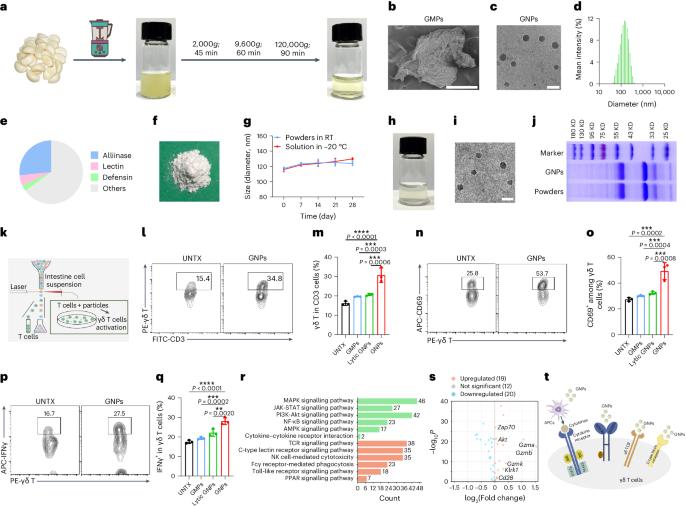
口服大蒜衍生纳米颗粒可通过诱导肠道IFNγ产生γδT细胞改善癌症免疫疗法。
以γ-δ(γδ)T 细胞为基础的癌症免疫疗法是一种前景广阔的癌症治疗方法。然而,由于这些细胞在体内的扩增和分化有限,它们的发展面临挑战。在这里,我们通过口服大蒜提取的纳米颗粒(GNPs)诱导了γδT细胞的内源性扩增和活化。我们发现,GNPs 能显著促进肠道内源性 γδ T 细胞的增殖和活化,从而产生大量干扰素-γ(IFNγ)。此外,经 GNP 处理的小鼠肠道γδ T 细胞中趋化因子 CXCR3 水平升高,这可促使它们从肠道迁移到肿瘤环境中。γδT细胞和IFNγ从肠道转移到肠道外的皮下肿瘤会重塑肿瘤免疫微环境,并与抗PD-L1协同作用,诱导强大的抗肿瘤免疫。我们的研究从机理上揭示了复杂的肠道-肿瘤相互作用组,为基于γδT细胞的免疫疗法提供了另一种方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


