Structural basis for activity switching in polymerases determining the fate of let-7 pre-miRNAs
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
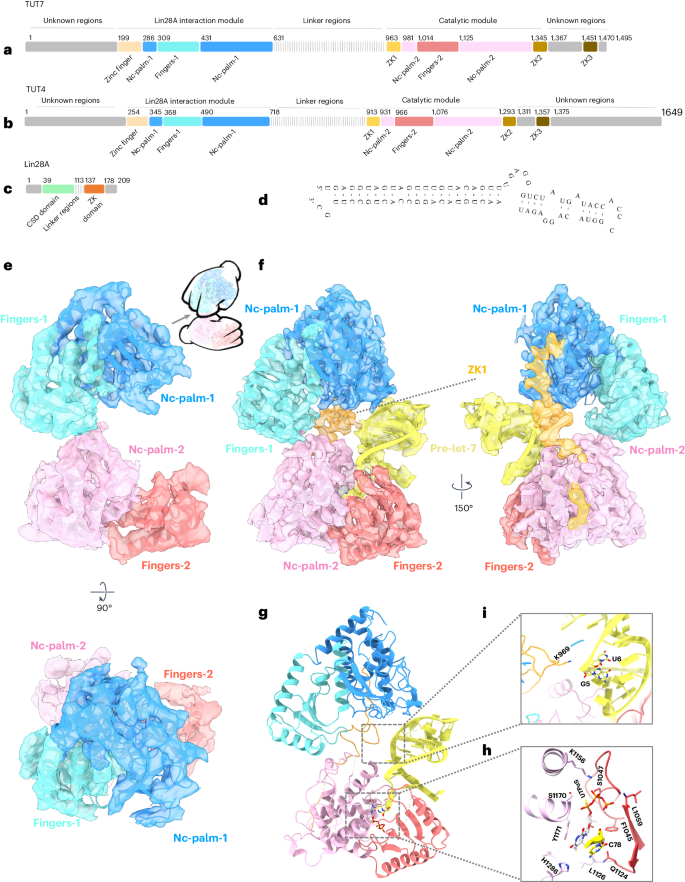
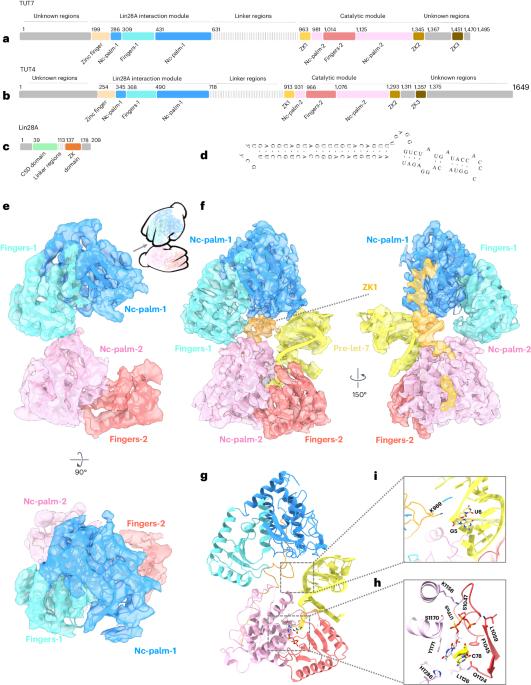
Tumor-suppressor let-7 pre-microRNAs (miRNAs) are regulated by terminal uridylyltransferases TUT7 and TUT4 that either promote let-7 maturation by adding a single uridine nucleotide to the pre-miRNA 3′ end or mark them for degradation by the addition of multiple uridines. Oligo-uridylation is increased in cells by enhanced TUT7/4 expression and especially by the RNA-binding pluripotency factor LIN28A. Using cryogenic electron microscopy, we captured high-resolution structures of active forms of TUT7 alone, of TUT7 plus pre-miRNA and of both TUT7 and TUT4 bound with pre-miRNA and LIN28A. Our structures reveal that pre-miRNAs engage the enzymes in fundamentally different ways depending on the presence of LIN28A, which clamps them onto the TUTs to enable processive 3′ oligo-uridylation. This study reveals the molecular basis for mono- versus oligo-uridylation by TUT7/4, as determined by the presence of LIN28A, and thus their mechanism of action in the regulation of cell fate and in cancer. Here, the authors show that cytoplasmic uridylyltransferases TUT7 and TUT4 bind let-7 pre-miRNA by alternative means in the absence and presence of Lin28A, which directly interacts with both RNA and enzyme to convert from a distributive to a processive mode of action.
决定 let-7 pre-miRNA 命运的聚合酶活性转换的结构基础。
抑制肿瘤的let-7前microRNA(miRNA)受末端尿苷酸转移酶TUT7和TUT4的调控,这两种酶要么通过在pre-miRNA 3'端添加单个尿苷核苷酸促进let-7的成熟,要么通过添加多个尿苷酸标记其降解。TUT7/4表达的增强,特别是RNA结合多能因子LIN28A的作用,使细胞中寡尿苷化增加。我们利用低温电子显微镜捕获了 TUT7 单独、TUT7 加上 pre-miRNA 以及 TUT7 和 TUT4 与 pre-miRNA 和 LIN28A 结合的高分辨率活性结构。我们的结构揭示出,pre-miRNA 与酶的结合方式根本不同,这取决于 LIN28A 的存在,LIN28A 将它们夹在 TUT 上,使 3' 寡核苷酸化成为可能。这项研究揭示了 LIN28A 的存在所决定的 TUT7/4 单尿嘧啶化与寡尿嘧啶化的分子基础,从而揭示了它们在细胞命运调控和癌症中的作用机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


