Neoantigen-specific cytotoxic Tr1 CD4 T cells suppress cancer immunotherapy
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
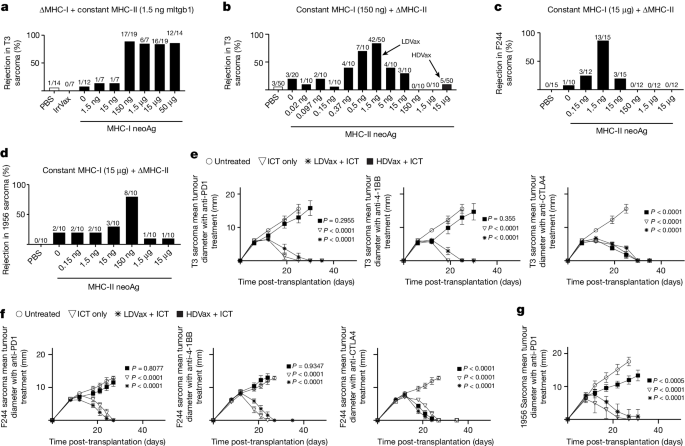
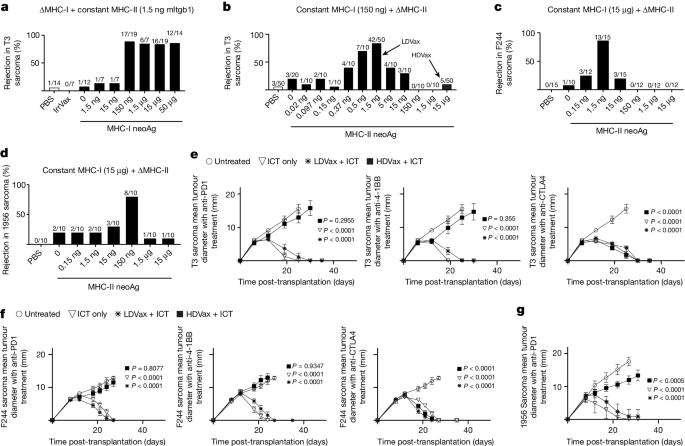
CD4+ T cells can either enhance or inhibit tumour immunity. Although regulatory T cells have long been known to impede antitumour responses1–5, other CD4+ T cells have recently been implicated in inhibiting this response6,7. Yet, the nature and function of the latter remain unclear. Here, using vaccines containing MHC class I (MHC-I) neoantigens (neoAgs) and different doses of tumour-derived MHC-II neoAgs, we discovered that whereas the inclusion of vaccines with low doses of MHC-II-restricted peptides (LDVax) promoted tumour rejection, vaccines containing high doses of the same MHC-II neoAgs (HDVax) inhibited rejection. Characterization of the inhibitory cells induced by HDVax identified them as type 1 regulatory T (Tr1) cells expressing IL-10, granzyme B, perforin, CCL5 and LILRB4. Tumour-specific Tr1 cells suppressed tumour rejection induced by anti-PD1, LDVax or adoptively transferred tumour-specific effector T cells. Mechanistically, HDVax-induced Tr1 cells selectively killed MHC-II tumour antigen-presenting type 1 conventional dendritic cells (cDC1s), leading to low numbers of cDC1s in tumours. We then documented modalities to overcome this inhibition, specifically via anti-LILRB4 blockade, using a CD8-directed IL-2 mutein, or targeted loss of cDC2/monocytes. Collectively, these data show that cytotoxic Tr1 cells, which maintain peripheral tolerance, also inhibit antitumour responses and thereby function to impede immune control of cancer. Type 1 regulatory T cells (Tr1) represent a major obstacle that compromises naturally occurring and therapeutically induced tumour-specific immunity.
新抗原特异性细胞毒性 Tr1 CD4 T 细胞抑制癌症免疫疗法。
CD4+ T 细胞可以增强或抑制肿瘤免疫。尽管人们早就知道调节性 T 细胞会阻碍抗肿瘤反应1-5,但最近又发现其他 CD4+ T 细胞与抑制这种反应有关6,7。然而,后者的性质和功能仍不清楚。在这里,我们利用含有 MHC I 类新抗原(neoAgs)和不同剂量的肿瘤衍生 MHC-II neoAgs 的疫苗发现,含有低剂量 MHC-II 限制肽的疫苗(LDVax)会促进肿瘤排斥反应,而含有高剂量相同 MHC-II neoAgs 的疫苗(HDVax)则会抑制排斥反应。对HDVax诱导的抑制性细胞进行鉴定后发现,它们是表达IL-10、颗粒酶B、穿孔素、CCL5和LILRB4的1型调节性T(Tr1)细胞。肿瘤特异性Tr1细胞抑制了抗PD1、LDVax或收养性转移肿瘤特异性效应T细胞诱导的肿瘤排斥反应。从机制上讲,HDVax诱导的Tr1细胞可选择性地杀死MHC-II肿瘤抗原递呈1型常规树突状细胞(cDC1s),从而导致肿瘤中cDC1s的数量减少。我们随后记录了克服这种抑制作用的方法,特别是通过抗LILRB4阻断、使用CD8定向IL-2静默素或靶向损失cDC2/单核细胞。这些数据共同表明,维持外周耐受性的细胞毒性Tr1细胞也会抑制抗肿瘤反应,从而起到阻碍癌症免疫控制的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


