Transition Metal-Driven Selectivity in Direct C−H Arylation of Imidazo[2,1-b]Thiazole
IF 2.5
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A selective direct arylation of the different Csp2-H bonds of imidazo[2,1-b]thiazole with (hetero) aryl halides can be achieved simply by switching from a palladium catalyst system to the use of stoichiometric amounts of copper. The observed selectivity, also rationalized by DFT calculations, can be explained by a change in the mechanistic pathways between electrophilic palladation and base-promoted C−H metalation.
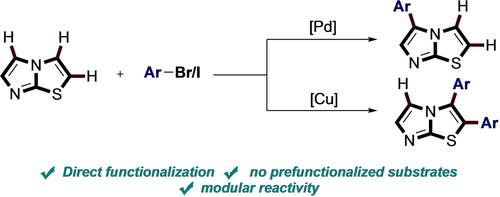
过渡金属驱动的咪唑并[2,1-b]噻唑直接 C-H 芳基化选择性。
只需将钯催化剂体系转换为使用等当量的铜,就能实现咪唑并[2,1-b]噻唑的不同 Csp2-H 键与(杂)芳基卤化物的选择性直接芳基化。观察到的选择性也可通过 DFT 计算得到合理解释,即亲电钯化和碱促进 C-H 金属化之间的机械路径发生了变化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ChemistryOpen
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
4.80
自引率
4.30%
发文量
143
审稿时长
1 months
期刊介绍:
ChemistryOpen is a multidisciplinary, gold-road open-access, international forum for the publication of outstanding Reviews, Full Papers, and Communications from all areas of chemistry and related fields. It is co-owned by 16 continental European Chemical Societies, who have banded together in the alliance called ChemPubSoc Europe for the purpose of publishing high-quality journals in the field of chemistry and its border disciplines. As some of the governments of the countries represented in ChemPubSoc Europe have strongly recommended that the research conducted with their funding is freely accessible for all readers (Open Access), ChemPubSoc Europe was concerned that no journal for which the ethical standards were monitored by a chemical society was available for such papers. ChemistryOpen fills this gap.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


