Development of a Mild and Efficient Process for Ir-Catalyzed N-Alkylation of 4-Bromopyridin-2-amine with a Primary Alcohol via Borrowing Hydrogen
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
We report herein the development and scale up of an Ir-catalyzed N-alkylation reaction between a 4-bromopyridin-2-amine (1) and (4-(5-(1,1-difluoroethyl)-1,2,4-oxadiazol-3-yl)bicyclo[2.2.2]octan-1-yl)methanol (2) proceeding via a borrowing hydrogen process. The traditional approach of alcohol oxidation followed by reductive amination posed challenges that are attributed to the poor nucleophilicity of the 2-aminopyridine derivative (1) resulting in lower isolated yields. Several catalysts and bases were evaluated for the successful N-alkylation of 1 with 2, and an Ir (III) catalyst in combination with LiOt-Bu as a base was found to provide optimal conversion. The borrowing hydrogen process was successfully demonstrated on a 1.5 kg scale and afforded >70% yield of 3 without the need for a sealed reactor or any other specialized equipment.
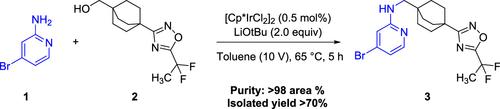
通过借用氢气开发温和高效的 4-溴吡啶-2-胺与伯醇的铱催化 N-烷基化工艺
我们在此报告 4-溴吡啶-2-胺 (1) 和 (4-(5-(1,1-二氟乙基)-1,2,4-恶二唑-3-基)双环[2.2.2]辛烷-1-基)甲醇 (2) 之间通过借氢过程进行的铱催化 N- 烷基化反应的开发和放大。由于 2- 氨基吡啶衍生物 (1) 的亲核性较差,导致分离产率较低,因此先醇氧化再还原胺化的传统方法面临挑战。为了成功地将 1 与 2 进行 N- 烷基化,对几种催化剂和碱进行了评估,发现 Ir (III) 催化剂结合 LiOt-Bu 作为碱可提供最佳转化率。借氢工艺在 1.5 千克的规模上得到了成功验证,3 的收率达到 70%,且无需密封反应器或任何其他专用设备。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


