Oxidative Photochemical Cyclisations to Access Spiroketals
IF 4.4
2区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The spiroketal moiety is an important substructure within many biological natural products. One method to access them is via the oxidative cyclisation of a pendant hydroxyl group on to a pre‐formed pyran. However applications of this methodology have been severely limited by requiring the use of toxic oxidants, such as lead (IV) tetraacetate or mercuric oxide. Herein we report a high yielding photochemical route to prepare complex spiroketals using an oxidative photochemical approach employing iodine monochloride and sodium acetate and demonstrate the methodology to the synthesis of a number of insect pheromones.
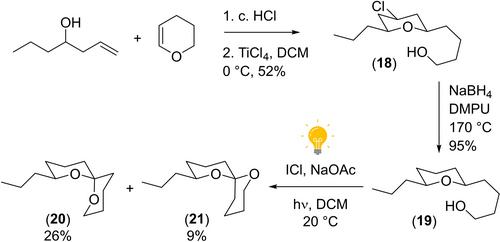
通过氧化光化学环化反应获得螺环酮类化合物
螺酮分子是许多生物天然产物中的重要子结构。获取它们的一种方法是将一个悬挂的羟基氧化环化到预先形成的吡喃上。然而,由于需要使用有毒的强氧化剂,如四乙酸铅或氧化汞,这种方法的应用受到严重限制。在此,我们报告了一种新颖、高产的光化学方法,即使用温和无毒的试剂,通过光化学氧化法制备复杂的螺酮,并演示了该方法合成多种昆虫信息素的过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Synthesis & Catalysis
化学-应用化学
CiteScore
9.40
自引率
7.40%
发文量
447
审稿时长
1.8 months
期刊介绍:
Advanced Synthesis & Catalysis (ASC) is the leading primary journal in organic, organometallic, and applied chemistry.
The high impact of ASC can be attributed to the unique focus of the journal, which publishes exciting new results from academic and industrial labs on efficient, practical, and environmentally friendly organic synthesis. While homogeneous, heterogeneous, organic, and enzyme catalysis are key technologies to achieve green synthesis, significant contributions to the same goal by synthesis design, reaction techniques, flow chemistry, and continuous processing, multiphase catalysis, green solvents, catalyst immobilization, and recycling, separation science, and process development are also featured in ASC. The Aims and Scope can be found in the Notice to Authors or on the first page of the table of contents in every issue.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


