Organocatalytic hydrogen bond donor/Lewis base (HBD/LB) synthesis and chiroptical properties of thiabridged [5]helicenes†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Thiabridged [5]helicenes are obtained from thioaryl-N-phthalimido benzo[a]phenothiazines using a hydrogen bond donor/Lewis base organocatalytic approach. Resolution of [5]helicenes using either (1S)-(−)-camphanic acid as a chiral auxiliary or CSP-HPLC is reported. Thiabridged [5]helicenes show an exceptional configurational stability with racemization energy barriers higher than 40 kcal mol−1. Electronic circular dichroism and TD-DFT calculations permit the assignment of the absolute configuration, demonstrating that the sign of optical rotation is not easily related to the M or P structure. Separated enantiomers show circularly polarized luminescence with high dissymmetry ratio.
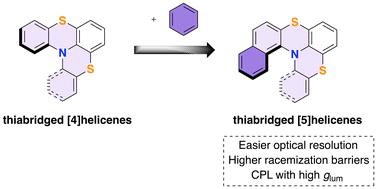
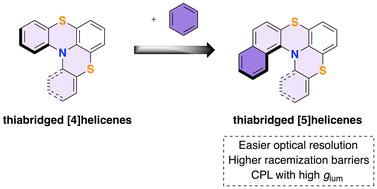
有机催化氢键供体/刘易斯碱(HBD/LB)合成及硫杂[5]螺旋烯的光电性质。
利用氢键供体/刘易斯碱有机催化法,从硫代芳基-N-邻苯二甲酰亚胺基苯并[a]吩噻嗪中获得了硫杂[5]庚烯。报告采用 (1S)-(-)-莰烷酸作为手性辅助剂或 CSP-HPLC 法解析了 [5]helicenes。硫杂[5]螺旋烯显示出优异的构型稳定性,外消旋化能垒高于 40 kcal mol-1。通过电子圆二色性和 TD-DFT 计算,可以确定绝对构型,证明光学旋转的符号与 M 或 P 结构的关系并不简单。分离的对映体显示出高不对称率的圆偏振发光。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


