Self-activated energy release cascade from anthracene-based solid-state molecular solar thermal energy storage systems
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We introduce donor-acceptor substituted anthracenes as effective molecular solar thermal energy storage compounds that operate exclusively in the solid state. The donor-acceptor anthracenes undergo a visible light-induced [4+4] cycloaddition reaction, producing metastable cycloadducts—dianthracenes with quaternary carbons—and storing photon energy. The triggered cycloreversion of dianthracenes to anthracenes discharges the stored energy as heat in the order of 100 kJ/mol (200 J/g). The series of compounds displays remarkable self-heating, or cascading heat release, upon the initial triggering. Such self-activated energy release is enabled by the large energy storage in dianthracenes, low activation energy for their thermal reversion, and effective heat transfer to unreacted molecules in the solid state. This process mirroring the self-ignition of fossil fuels opens up opportunities to use dianthracenes as effective and renewable solid-state fuels that can release energy rapidly and completely upon initial activation.
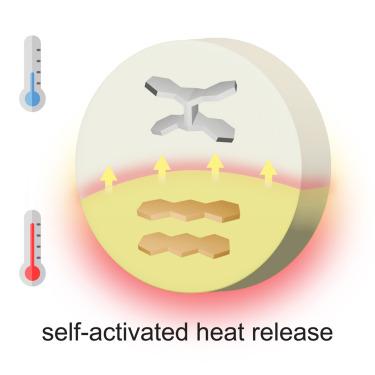
蒽基固态分子太阳能热储存系统的自激活能量释放级联
我们将供体-受体取代的蒽作为有效的分子太阳能热能储存化合物介绍给大家,这种化合物只在固态下工作。供体-受体蒽发生可见光诱导的[4+4]环加成反应,生成具有季碳的可转移环加成物--二蒽,并储存光子能量。二蒽与蒽的触发环化反应将储存的能量以热量的形式释放出来,热量约为 100 kJ/mol(200 J/g)。这一系列化合物在最初触发时显示出显著的自加热或级联热释放。这种自激活能量释放得益于二蒽的巨大能量储存、其热还原的低活化能以及固态中未反应分子的有效热传导。这一过程与化石燃料的自燃过程如出一辙,为将二蒽用作有效的可再生固态燃料提供了机会,这种燃料在初始活化时就能快速、完全地释放能量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


