Selective Nitrate Synthesis by Plasma/Liquid Water Reaction: An Enhanced Oxidative Strength Mechanism in Nitrogen Discharge
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
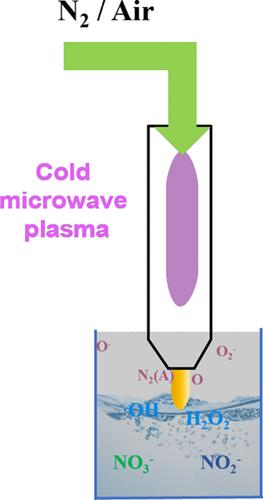
Plasma/liquid (P/L) reaction is an emerging green chemical process for nitrogen fixation into ammonia or nitrogen oxides directly from air and water. On the other hand, the P/L synthesis is often limited by product nonselectivity. Mechanistic understanding is essential to achieve selective nitrate synthesis in P/L reactions, which would also be profitable in practice. In this study, selective NO3– synthesis from nitrogen gas and liquid water was achieved via a cold microwave plasma (CMP) at ambient temperature and pressure. The product yield and selectivity were found to be controlled by tuning the types of discharge gases as well as the treatment time. The results demonstrate that NO3– was exclusively produced in nitrogen discharge at an extended treatment time of 20 min. Various spectroscopy characterizations and reactive nitrogen or oxygen species measurements were conducted to understand the underlying mechanism. In the nitrogen plasma, the abundant metastable N2(A) species were suggested to be responsible for the ample hydroxyl radicals generated from water, which in turn plays a key role for the high NO3– selectivity. The results in this work not only give insight into the nitrogen fixation process in plasma and liquid water systems but also have a more general relevance to the understanding of P/L based chemical transformation.
等离子体/液态水反应的选择性硝酸盐合成:氮气排放中的氧化强度增强机制
等离子体/液体(P/L)反应是一种新兴的绿色化学工艺,可直接从空气和水中固氮为氨或氮氧化物。另一方面,P/L 合成往往受限于产物的非选择性。要想在 P/L 反应中实现选择性硝酸盐合成,并在实践中获利,对机理的理解至关重要。本研究在常温常压下通过冷微波等离子体(CMP)实现了从氮气和液态水中选择性合成 NO3-。通过调整放电气体的种类和处理时间,可以控制产品的产量和选择性。结果表明,在延长 20 分钟的处理时间后,氮气放电只产生 NO3-。为了解其基本机制,还进行了各种光谱特性分析和活性氮或氧物种测量。在氮等离子体中,丰富的瞬态 N2(A) 物种被认为是水产生大量羟基自由基的原因,而羟基自由基又是高 NO3- 选择性的关键因素。这项工作的结果不仅有助于深入了解等离子体和液态水系统中的固氮过程,而且对理解基于 P/L 的化学转化具有更普遍的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


