Effect of Solution Conditions and Applied Potential on Ion Transport in TiO2 Nanopores
IF 7.4
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
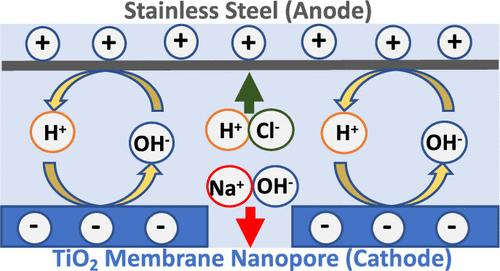
This study investigated the material and ion transport properties of TiO2 nanopores as a function of solution conditions and applied electrode potentials. Zeta potential measurements revealed that the TiO2 surface charge was highly dependent on solution conditions, which was attributed to protonation/deprotonation of surface functional groups and adsorption of ions. Ion rejection followed the absolute magnitude of the membrane surface charge and was pH-dependent, reflecting the amphoteric nature of TiO2. The rejection of NaCl was approximately symmetrical about the point of zero charge of the membrane, with the highest rejection at acidic and basic conditions. Specific adsorption of SO42– and Mg2+ under acidic and basic conditions, respectively, neutralized the membrane charge and significantly reduced ion rejection. A mathematical transport model was fit to experimental data, and the model-determined membrane charge densities as a function of solution conditions agreed with experimental zeta potential measurements. Model results also revealed that rejection was primarily attributed to the Donnan exclusion mechanism. The application of both anodic and cathodic potentials directly to the TiO2 membrane caused permselective transport under specific solution conditions.
溶液条件和应用电位对二氧化钛纳米孔中离子传输的影响
本研究探讨了二氧化钛纳米孔的材料和离子传输特性与溶液条件和应用电极电位的函数关系。Zeta 电位测量显示,TiO2 表面电荷与溶液条件高度相关,这归因于表面官能团的质子化/去质子化和离子吸附。离子排斥与膜表面电荷的绝对值有关,并与 pH 值相关,这反映了二氧化钛的两性性质。对 NaCl 的抑制作用与膜的零电荷点大致对称,在酸性和碱性条件下抑制作用最强。在酸性和碱性条件下,分别对 SO42- 和 Mg2+ 的特定吸附中和了膜电荷,大大降低了离子抑制作用。根据实验数据拟合了一个数学传输模型,模型确定的膜电荷密度与溶液条件的函数关系与实验的 zeta 电位测量结果一致。模型结果还显示,排斥主要归因于唐南排除机制。在特定的溶液条件下,对二氧化钛膜直接施加阳极电位和阴极电位会引起全选择性迁移。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS ES&T engineering
ENGINEERING, ENVIRONMENTAL-
CiteScore
8.50
自引率
0.00%
发文量
0
期刊介绍:
ACS ES&T Engineering publishes impactful research and review articles across all realms of environmental technology and engineering, employing a rigorous peer-review process. As a specialized journal, it aims to provide an international platform for research and innovation, inviting contributions on materials technologies, processes, data analytics, and engineering systems that can effectively manage, protect, and remediate air, water, and soil quality, as well as treat wastes and recover resources.
The journal encourages research that supports informed decision-making within complex engineered systems and is grounded in mechanistic science and analytics, describing intricate environmental engineering systems. It considers papers presenting novel advancements, spanning from laboratory discovery to field-based application. However, case or demonstration studies lacking significant scientific advancements and technological innovations are not within its scope.
Contributions containing experimental and/or theoretical methods, rooted in engineering principles and integrated with knowledge from other disciplines, are welcomed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


