Selectivity of N2–CH4 Mixed Clathrate Hydrates: A Grand Canonical Monte Carlo Study
IF 2.9
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this article, we report the results of a grand canonical Monte Carlo simulation study aiming at characterizing the competitive trapping of N2 and CH4 molecules into the same clathrate hydrate structure. Simulations have been performed at temperature conditions typical of those encountered at the surface of, e.g., Titan, and in accordance with recent experiments. Various compositions of the fluid in contact with the clathrate hydrate phase, i.e., various nitrogen/methane ratios, have been considered in the simulations. The results of these simulations reveal that, although the ratio between the number of enclathrated methane and that of nitrogen molecules strongly varies with the composition of the fluid phase, the clathrate hydrate lattice remains always selective for methane. This means that the relative proportion of methane with respect to nitrogen is always larger in the clathrate phase than in the fluid phase, with the mixed clathrate hydrate thus acting as a methane concentrator, irrespective of the fluid composition. These features indicate that the formation of clathrate hydrates can strongly influence the composition of the nitrogen–methane fluid phase, which may thus be impoverished in methane, as already inferred from the experimental results.
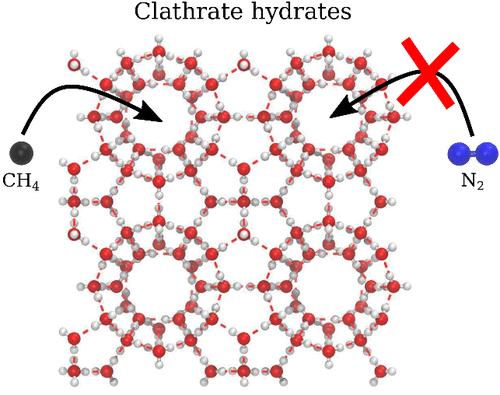
N2-CH4 混合水合物的选择性:大规范蒙特卡洛研究
本文报告了大规范蒙特卡洛模拟研究的结果,该研究旨在描述 N2 和 CH4 分子在同一凝块水合物结构中的竞争性捕获。模拟是在土卫六等表面的典型温度条件下进行的,与最近的实验相符。模拟中考虑了与凝块水合物相接触的流体的各种成分,即各种氮气/甲烷比例。模拟结果表明,虽然甲烷和氮分子的附着数量比例随流体相的成分而强烈变化,但水合物晶格始终对甲烷具有选择性。这意味着,甲烷与氮气的相对比例在克拉液相中总是大于在流体相中,因此,无论流体成分如何,混合克拉液水合物都起着甲烷浓缩器的作用。这些特征表明,凝块水合物的形成会对氮-甲烷流体相的组成产生很大影响,因此,正如实验结果所推断的那样,流体相中的甲烷可能会变得贫乏。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Earth and Space Chemistry
Earth and Planetary Sciences-Geochemistry and Petrology
CiteScore
5.30
自引率
11.80%
发文量
249
期刊介绍:
The scope of ACS Earth and Space Chemistry includes the application of analytical, experimental and theoretical chemistry to investigate research questions relevant to the Earth and Space. The journal encompasses the highly interdisciplinary nature of research in this area, while emphasizing chemistry and chemical research tools as the unifying theme. The journal publishes broadly in the domains of high- and low-temperature geochemistry, atmospheric chemistry, marine chemistry, planetary chemistry, astrochemistry, and analytical geochemistry. ACS Earth and Space Chemistry publishes Articles, Letters, Reviews, and Features to provide flexible formats to readily communicate all aspects of research in these fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


