On-surface Synthesis of Multiple Non-benzenoid Carbohelicenes Fused with Fluorene Unit(s)
Abstract
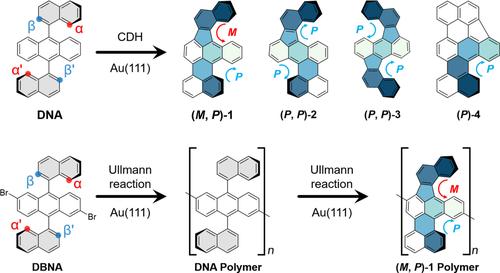
Carbohelicenes have garnered considerable attention for their inherent chirality and structural flexibility. Increasing multi-helicity and incorporating non-six-membered rings to substitute benzenoid rings within helicenes are effective strategies for introducing unique photoelectric properties. Despite the disclosure of numerous helicenes, the inaccessible precursors and the lack of synthetic routes pose a challenge in achieving desired helicene structures fused with non-benzenoid rings. Herein, we report the synthesis of multiple non-benzenoid carbohelicenes fused with fluorene unit(s) through intramolecular cyclodehydrogenation of 9,10-di(naphthalen-1- yl)anthracene on Au(111) surface. Two potential cyclodehydrogenation manners between naphthyl and anthracene lead to the formation of fluorene-fused [5]helicene and [4]helicene moiety. Consequently, a total of four stable products were observed. The atomic topographies of products are characterized by bond-resolving scanning tunneling microscopy. The chiral helicity of targeted products can be switched by tip manipulation. Density-functional-theory calculations unveils the reaction pathway of four products. The comparative analysis of their respective energy barriers exhibits a correlation with the experimentally determined yields. Furthermore, we synthesize the polymer chains incorporating non-benzenoid carbohelicenes via the Ullmann reaction of 2,6-dibromo-9,10-di(1-naphthyl)anthracene precursors. Our work proposes a synthetic methodology for several novel helicene-like structures fused with fluorene units and the polymer bearing helicene subunits, thus highlighting the immense potential of these compounds in the application fields of luminescent electronic devices.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


