Synthesis, characterization, and evaluation of improved electrochemical performance of vanadium and zinc co-doped Ni-rich oxide cathode materials: experimental and first-principles study
Abstract
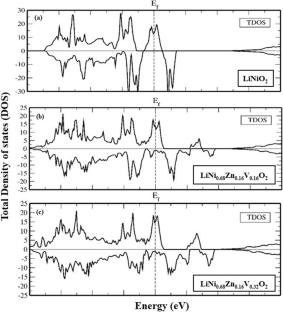
Ni-rich transition metal-based oxide materials have excellent electrochemical properties that make their specific discharge capacity and voltages suitable as cathodes in Li-ion batteries. The current investigation uses solid-state synthesis to create a variety of Ni-rich metal oxide cathode materials, including vanadium (V) and zinc (Zn) co-doped LiNiO2. XRD analysis demonstrates that the synthesized materials exhibit a stable hexagonal structure with an R3m space group. Scanning electron micrographs (SEM) reveal the production of well-shaped particles with different doping concentrations, while energy-dispersive spectroscopy (EDS) mapping validates the presence of Ni, V, Zn, and O with the appropriate compositions. Selected area electron diffraction (SEAD) and transmission electron microscopy (TEM) confirm that the synthesized polycrystalline LiNi0.80Zn0.06V0.14O2 cathode material has crystals organized in a hexagonal phase. Structural properties are also calculated by density functional theory (DFT) using Wien2K code. The spin-polarised electronic band structures and density of states (DOS) are calculated for all given compounds showing the ferromagnetic nature. The theoretical discharge capacity and intercalation voltages are determined by adding up the total energies of the optimized compounds. It claimed that LiNi0.52Zn0.16V0.32 O2 has a discharge capacity of 48–246 mAhg−1 with an intercalation voltage of 5.77–3.35 V, proving significant improvement in the redox properties. Theoretical calculations and experimental results both examined Ni-rich co-doped V and Zn transition metal oxides as potential materials for the fabrication of coin cells in future batteries.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


