CO2-to-methanol electroconversion on a molecular cobalt catalyst facilitated by acidic cations
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
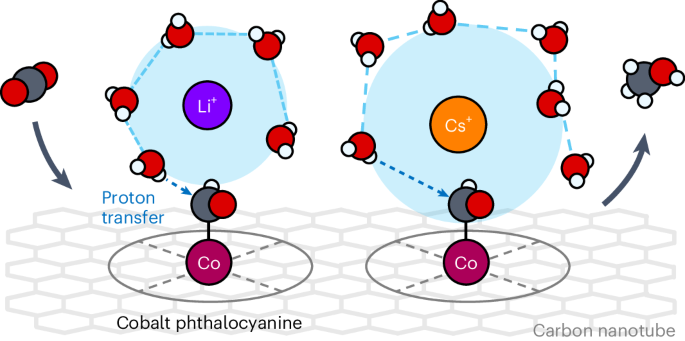
The crucial role of electrolyte cations in CO2 electroreduction has received intensive attention. One prevailing theory is that through electrostatic interactions or direct coordination, larger cations such as Cs+ can better stabilize the key intermediate species for CO and multicarbon (C2+) product generation, for example, on silver and copper, respectively. Here we show that smaller, more acidic alkali metal cations greatly enhance CO2-to-methanol conversion kinetics (Li+ > Na+ > K+ > Cs+) on an immobilized molecular cobalt catalyst, unlike the trend observed for CO and C2+. Through electrokinetic analyses and kinetic isotope effect studies along with computational investigations, we show that the hydration shell of a cation serves as a proton donor in the rate-determining protonation step of adsorbed CHO where acidic cations promote the proton-coupled electron transfer. This study reveals the promotional effect of cation solvation environment on CO2 electroreduction beyond the widely acknowledged stabilizing effect of cations. The non-innocence of electrolyte cations in the activity of CO2 reduction has been widely acknowledged, although these complex effects are not fully understood. Here, opposite to the trend observed with Cu and Ag surfaces, smaller alkali cations greatly enhance CO2-to-methanol conversion on a cobalt molecular electrocatalyst.
酸性阳离子促进分子钴催化剂上的二氧化碳-甲醇电转化
电解质阳离子在二氧化碳电还原中的关键作用受到了广泛关注。一种流行的理论认为,通过静电相互作用或直接配位,较大的阳离子(如 Cs+)可以更好地稳定 CO 和多碳(C2+)产物生成的关键中间物种,例如分别稳定在银和铜上。在这里,我们表明,在固定化分子钴催化剂上,更小、酸性更强的碱金属阳离子(Li+ > Na+ > K+ > Cs+)能大大提高 CO2 到甲醇的转化动力学,这与 CO 和 C2+ 的转化趋势不同。通过电动力学分析和动力学同位素效应研究以及计算研究,我们发现在吸附 CHO 的决定速率的质子化步骤中,阳离子的水合壳可作为质子供体,其中酸性阳离子可促进质子耦合电子转移。这项研究揭示了阳离子溶解环境对 CO2 电还原的促进作用,而不仅仅是公认的阳离子稳定作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


