Magnetic-field-driven targeting of exosomes modulates immune and metabolic changes in dystrophic muscle
IF 38.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
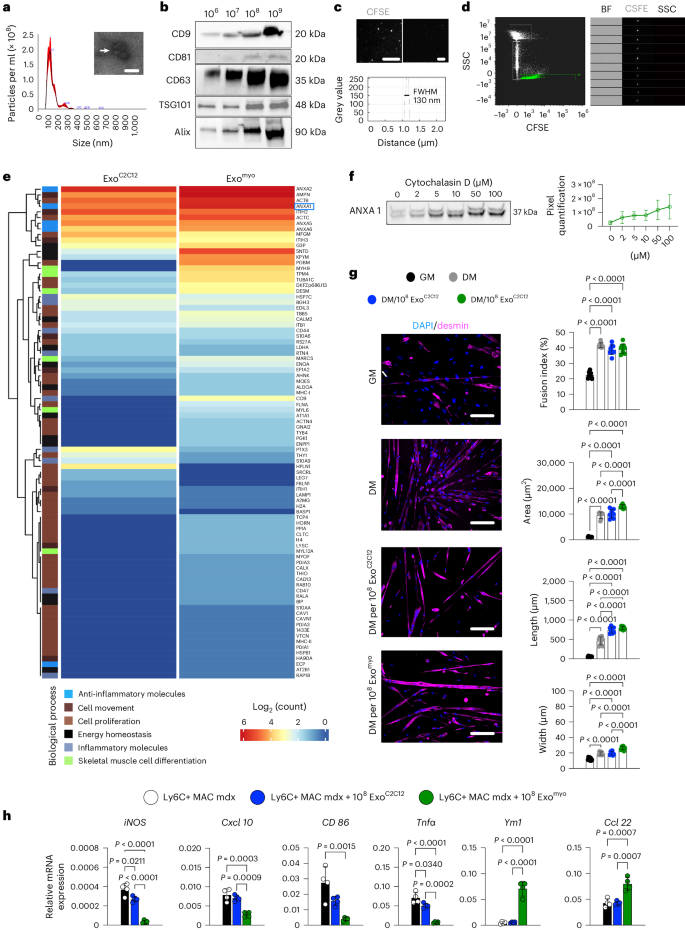
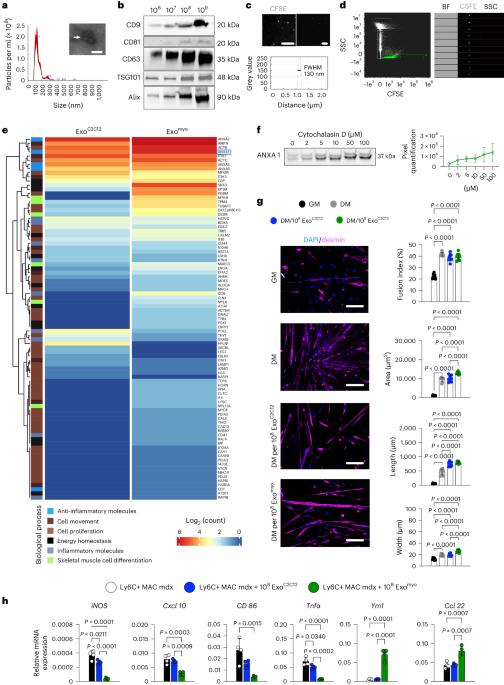
Exosomes are promising therapeutics for tissue repair and regeneration to induce and guide appropriate immune responses in dystrophic pathologies. However, manipulating exosomes to control their biodistribution and targeting them in vivo to achieve adequate therapeutic benefits still poses a major challenge. Here we overcome this limitation by developing an externally controlled delivery system for primed annexin A1 myo-exosomes (Exomyo). Effective nanocarriers are realized by immobilizing the Exomyo onto ferromagnetic nanotubes to achieve controlled delivery and localization of Exomyo to skeletal muscles by systemic injection using an external magnetic field. Quantitative muscle-level analyses revealed that macrophages dominate the uptake of Exomyo from these ferromagnetic nanotubes in vivo to synergistically promote beneficial muscle responses in a murine animal model of Duchenne muscular dystrophy. Our findings provide insights into the development of exosome-based therapies for muscle diseases and, in general, highlight the formulation of effective functional nanocarriers aimed at optimizing exosome biodistribution. Exosome targeting for therapeutic needs remains a challenge. Here, the authors show that ferromagnetic-nanotube-passivated exosomes promote the transition of proinflammatory macrophages to an anti-inflammatory state and myogenic maturation of dystrophic muscle progenitors in a murine model.
磁场驱动的外泌体靶向调节肌营养不良症肌肉的免疫和代谢变化
外泌体是一种很有前景的组织修复和再生疗法,可诱导和引导适当的免疫反应,治疗组织萎缩性病变。然而,如何操纵外泌体以控制其生物分布并将其用于体内靶向以获得充分的治疗效果仍是一大挑战。在这里,我们通过开发一种外部控制的引物附件素 A1 肌外体(Exomyo)递送系统来克服这一限制。通过将Exomyo固定在铁磁性纳米管上,实现了有效的纳米载体,从而利用外部磁场通过全身注射将Exomyo可控地输送到骨骼肌并定位。肌肉层面的定量分析显示,在杜氏肌营养不良症的小鼠动物模型中,巨噬细胞主导了这些铁磁纳米管对Exomyo的吸收,从而协同促进了有益的肌肉反应。我们的研究结果为开发基于外泌体的肌肉疾病疗法提供了启示,总体而言,突出了旨在优化外泌体生物分布的有效功能纳米载体的配方。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


