An in vitro study of the impact of IL-17A and IL-22 on ciliogenesis in nasal polyps epithelium via the Hippo-YAP pathway
IF 11.4
1区 医学
Q1 ALLERGY
引用次数: 0
Abstract
Background
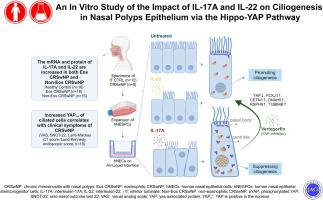
Cilia loss and impaired motile ciliary functions are among the typical pathological features of chronic rhinosinusitis with nasal polyps (CRSwNP). IL17A and IL22 are the canonical cytokines of type 3 inflammation, exhibiting similar functional effects on epithelial cells. In this study, we sought to examine the effects of IL17A and IL22 on ciliated cells and investigate the potential involvement of Hippo-YAP signaling in their influence on ciliogenesis.
Methods
We assessed both the mRNA and protein expression levels of IL17A and IL22 in nasal tissues obtained from patients with CRSwNP and compared them to those from healthy controls. To further explore the impact of IL17A and IL22, we established a primary human nasal epithelial cell model using different concentrations (2 ng/mL, 10 ng/mL, 50 ng/mL) for a duration of 28 days in an air-liquid interface culture. Additionally, we employed the inhibitor verteporfin to investigate whether IL17A and IL22 exert their effects on ciliated cells via the Hippo-YAP pathway.
Results
The mRNA and protein levels of IL17A and IL22 in CRSwNP were significantly higher than those in healthy controls, revealing a robust correlation between IL17A and IL22. YAP was highly expressed in the nucleus of ciliated cells in CRSwNP and displayed a positive correlation with clinical symptoms. Both IL17A and IL22 were found to reduce the number of ciliated cells. IL17A, but not IL22, suppressed ciliogenesis by disrupting the proper development and docking of the basal body of ciliated cells, resulting in motile ciliary dysfunctions. Furthermore, the expression of YAP within the nucleus of ciliated cells gradually declined as these cells reached the final stage of differentiation. However, this process was obstructed by IL17A only. YAP inhibitors, such as verteporfin, markedly reversed the effects of IL17A by increasing the proportion of ciliated cells, suppressing nuclear YAP expression in these cells, and enhancing ciliary beating frequency.
Conclusions
Both IL17A and IL22 are overexpressed in nasal epithelium of CRSwNP, which is associated with the impairment of epithelial cell differentiation. Furthermore, IL17A has been shown to exert a disruptive effect on morphogenesis of motile cilia via activation of YAP.
关于 IL-17A 和 IL-22 通过 Hippo-YAP 通路影响鼻息肉上皮细胞纤毛生成的体外研究
背景:纤毛脱落和纤毛运动功能受损是慢性鼻炎伴鼻息肉(CRSwNP)的典型病理特征之一。白细胞介素-17A(IL-17A)和白细胞介素-22(IL-22)是3型炎症的典型细胞因子,对上皮细胞有类似的功能影响。在本研究中,我们试图研究 IL-17A 和 IL-22 对纤毛细胞的影响,并探讨 Hippo-Yes-associated protein(YAP)信号转导可能参与它们对纤毛生成的影响:我们评估了 CRSwNP 患者鼻腔组织中 IL-17A 和 IL-22 的 mRNA 和蛋白表达水平,并与健康对照组进行了比较。为了进一步探讨 IL-17A 和 IL-22 的影响,我们建立了一个原代人鼻上皮细胞(hNEC)模型,在气液界面(ALI)培养中使用不同浓度(2 ng/mL、10 ng/mL、50 ng/mL),持续 28 天。此外,我们还使用抑制剂verteporfin(VP)来研究IL-17A和IL-22是否通过Hippo-YAP途径对纤毛细胞产生影响:结果:CRSwNP中IL-17A和IL-22的mRNA和蛋白水平均显著高于健康对照组,显示IL-17A和IL-22之间存在密切的相关性。YAP 在 CRSwNP 的纤毛细胞核中高表达,并与临床症状呈正相关。研究发现,IL-17A 和 IL-22 都能减少纤毛细胞的数量。IL-17A 而非 IL-22 通过破坏纤毛细胞基底体的正常发育和对接来抑制纤毛的生成,从而导致纤毛运动功能障碍。此外,纤毛细胞分化到最后阶段时,细胞核内 YAP 的表达逐渐减少。然而,这一过程只受到 IL-17A 的阻碍。YAP抑制剂(如Verteporfin)通过增加纤毛细胞的比例、抑制这些细胞核内YAP的表达和提高纤毛跳动频率,明显逆转了IL-17A的影响:结论:IL-17A 和 IL-22 在 CRSwNP 鼻上皮细胞中均过度表达,这与上皮细胞分化障碍有关。结论:IL-17A 和 IL-22 在 CRSwNP 鼻上皮细胞中过度表达,这与上皮细胞分化障碍有关。此外,IL-17A 还通过激活 YAP 对运动纤毛的形态发生产生破坏作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
25.90
自引率
7.70%
发文量
1302
审稿时长
38 days
期刊介绍:
The Journal of Allergy and Clinical Immunology is a prestigious publication that features groundbreaking research in the fields of Allergy, Asthma, and Immunology. This influential journal publishes high-impact research papers that explore various topics, including asthma, food allergy, allergic rhinitis, atopic dermatitis, primary immune deficiencies, occupational and environmental allergy, and other allergic and immunologic diseases. The articles not only report on clinical trials and mechanistic studies but also provide insights into novel therapies, underlying mechanisms, and important discoveries that contribute to our understanding of these diseases. By sharing this valuable information, the journal aims to enhance the diagnosis and management of patients in the future.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


