Membrane Disruption-Enhanced Photodynamic Therapy against Gram-Negative Bacteria by a Peptide-Photosensitizer Conjugate
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Photodynamic therapy (PDT) emerges as a promising strategy for combating bacteria with minimal drug resistance. However, a significant hurdle lies in the ineffectiveness of most photosensitizers against Gram-negative bacteria, primarily attributable to their characteristic impermeable outer membrane (OM) barrier. To tackle this obstacle, we herein report an amphipathic peptide-photosensitizer conjugate (PPC) with intrinsic outer membrane disruption capability to enhance PDT efficiency against Gram-negative bacteria. PPC is constructed by conjugating a hydrophilic ultrashort cationic peptide to a hydrophobic photosensitizer. PPC could efficiently bind to the OM of Gram-negative bacteria through electrostatic adsorption, and subsequently disrupt the structural integrity of the OM. Mechanistic investigations revealed that PPC triggers membrane disruption by binding to both lipopolysaccharide (LPS) and phospholipid leaflet in the OM, enabling effective penetration of PPC into the Gram-negative bacteria interior. Upon light irradiation, PPC inside bacteria generates singlet oxygen not only to effectively decrease the survival of Gram-negative bacteria P. aeruginosa and E. coli to nearly zero in vitro, but also successfully cure the full-thickness skin infection and bacterial keratitis (BK) induced by P. aeruginosa in animal models. Thus, this study provides a broad-spectrum antibacterial phototherapeutic design strategy by the synergistic action of membrane disruption and PDT to combat Gram-negative bacteria.
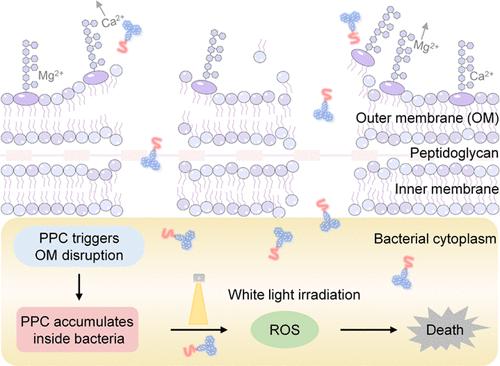
肽-光敏剂共轭物对革兰氏阴性细菌的膜破坏增强型光动力疗法
光动力疗法(PDT)是一种很有前景的抗菌策略,可将耐药性降至最低。然而,大多数光敏剂对革兰氏阴性菌无效是一个重大障碍,这主要是由于革兰氏阴性菌特有的不渗透外膜(OM)屏障。为了解决这一障碍,我们在此报告了一种具有内在外膜破坏能力的两性肽-光敏剂共轭物(PPC),以提高针对革兰氏阴性菌的光导治疗效率。PPC 由亲水性超短阳离子肽与疏水性光敏剂共轭而成。PPC 可通过静电吸附有效地与革兰氏阴性细菌的细胞膜结合,进而破坏细胞膜的结构完整性。机理研究发现,PPC 通过与 OM 中的脂多糖(LPS)和磷脂小叶结合引发膜破坏,从而使 PPC 有效渗透到革兰氏阴性细菌内部。经光照射后,细菌内部的 PPC 会产生单线态氧,不仅能有效降低革兰氏阴性菌铜绿假单胞菌和大肠杆菌在体外的存活率至近乎零,还能成功治愈铜绿假单胞菌在动物模型中诱发的全厚皮肤感染和细菌性角膜炎(BK)。因此,这项研究提供了一种广谱抗菌光疗设计策略,通过膜破坏和光动力疗法的协同作用来对抗革兰氏阴性菌。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


